Final Exam Practice 2016 (MC)
... d) There are too many electrons in this diagram. The lone pair on carbon should instead be a double bond with one of oxygen’s lone pairs. 23. The molecules CO2 and SO2 have very similar formulas yet make a different shape. What is different about their Lewis structures that give them a different sha ...
... d) There are too many electrons in this diagram. The lone pair on carbon should instead be a double bond with one of oxygen’s lone pairs. 23. The molecules CO2 and SO2 have very similar formulas yet make a different shape. What is different about their Lewis structures that give them a different sha ...
Second Semester Extra Review
... List some ways to increase the yield of products. 4. When will the pressure NOT affect an equilibrium system? Chapter 13 1. Define: a) Solute b) Solvent c) Alloy 2. What is an electrolyte? Give an example. 3. What are the three ways to increase the rate of dissolving? 4. How many grams of potassium ...
... List some ways to increase the yield of products. 4. When will the pressure NOT affect an equilibrium system? Chapter 13 1. Define: a) Solute b) Solvent c) Alloy 2. What is an electrolyte? Give an example. 3. What are the three ways to increase the rate of dissolving? 4. How many grams of potassium ...
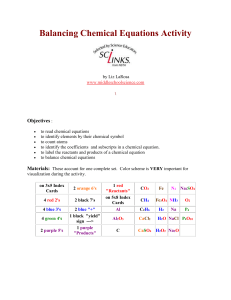
Balancing Chemical Equations Activity by Liz LaRosa www
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
Chapter 7: Solutions
... NaCl (aq) + KNO3 (aq) → NaNO3 (aq) + KCl (aq) IMPORTANT: If both of the “possible” products are water soluble, then no reaction occurred. There were solvated cations and anions in each the two solutions before mixing, then the solutions were mixed and the cations and anions remained solvated in the ...
... NaCl (aq) + KNO3 (aq) → NaNO3 (aq) + KCl (aq) IMPORTANT: If both of the “possible” products are water soluble, then no reaction occurred. There were solvated cations and anions in each the two solutions before mixing, then the solutions were mixed and the cations and anions remained solvated in the ...
Chemical Equations and Reaction Stoichiometry
... More Problems?? __NH3 + __O2 __NO + __H2O • How many grams of NO can be produced from 17.80 grams of O2? NH3 is in excess. • How many molecules of NH3 are required to produce 7.31 10-10 grams of H2O? ...
... More Problems?? __NH3 + __O2 __NO + __H2O • How many grams of NO can be produced from 17.80 grams of O2? NH3 is in excess. • How many molecules of NH3 are required to produce 7.31 10-10 grams of H2O? ...
periodic table - Mesa Community College
... OBJECTIVES: To develop the skills needed to write correct formulas from names and correct names from formulas. DISCUSSION: In the course of your study of chemistry you will encounter numerous compounds that you will have to recognize by formula and/or name. Either you are going to have to spend a gr ...
... OBJECTIVES: To develop the skills needed to write correct formulas from names and correct names from formulas. DISCUSSION: In the course of your study of chemistry you will encounter numerous compounds that you will have to recognize by formula and/or name. Either you are going to have to spend a gr ...
Properties of Solutions: Electrolytes and Non
... to blot the probe tip dry—however, the directions also remind them that they do not need to blot dry the inside of the hole containing the graphite electrodes. It is cumbersome to do so, and leaving a drop or two of distilled water does not significantly dilute the next sample. 7. Using the stored c ...
... to blot the probe tip dry—however, the directions also remind them that they do not need to blot dry the inside of the hole containing the graphite electrodes. It is cumbersome to do so, and leaving a drop or two of distilled water does not significantly dilute the next sample. 7. Using the stored c ...
Total Dissolved Solids
... to blot the probe tip dry—however, the directions also remind them that they do not need to blot dry the inside of the hole containing the graphite electrodes. It is cumbersome to do so, and leaving a drop or two of distilled water does not significantly dilute the next sample. 7. Using the stored c ...
... to blot the probe tip dry—however, the directions also remind them that they do not need to blot dry the inside of the hole containing the graphite electrodes. It is cumbersome to do so, and leaving a drop or two of distilled water does not significantly dilute the next sample. 7. Using the stored c ...
Chemical Bonds
... than that of bonded species) • Bond formation is accompanied by rearrangement of valence electrons – complete transfer of electrons – formation of ions (ionic bonding) – sharing of electrons – formation of molecules ...
... than that of bonded species) • Bond formation is accompanied by rearrangement of valence electrons – complete transfer of electrons – formation of ions (ionic bonding) – sharing of electrons – formation of molecules ...
Chem Final Study Guide Energy How much heat energy must be
... 67) What primarily determines the pH of a solution? a) Concentration of hydrodium/hydrogen ions 68) What is the pH of a solution that has an OH- ion concentration of 1× 10-3 mole per liter? Is it an acid or base? a) pH of 11, base 69) What is the H+ of an HCl solution if the pH is measured to be 4? ...
... 67) What primarily determines the pH of a solution? a) Concentration of hydrodium/hydrogen ions 68) What is the pH of a solution that has an OH- ion concentration of 1× 10-3 mole per liter? Is it an acid or base? a) pH of 11, base 69) What is the H+ of an HCl solution if the pH is measured to be 4? ...
Water
... When acid is half dissociated then the ratio is 1, at the point pKa=pH because the log of zero is one. Slide 19- Titration Curves: You can force acid and base into different configuration. Look at Bottom Figure Take acetic acid and add OH- equivalents (or adding base). The carboxyl group is go ...
... When acid is half dissociated then the ratio is 1, at the point pKa=pH because the log of zero is one. Slide 19- Titration Curves: You can force acid and base into different configuration. Look at Bottom Figure Take acetic acid and add OH- equivalents (or adding base). The carboxyl group is go ...
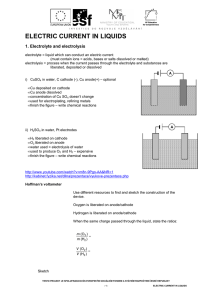
ELECTRIC CURRENT IN LIQUIDS
... R is directly/indirectly proportional to temperature, because ................................................................... ...
... R is directly/indirectly proportional to temperature, because ................................................................... ...
Practice Test 2
... The correct complete ionic equation for the reaction that occurs when aqueous solutions of Ca(NO3)2 and Na2CO3 are mixed is A) Ca(NO3)2(aq) + Na2CO3(aq) ----> CaCO3(s) + 2 NaNO3(aq) B) Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + CO32-(aq) ----> CaCO3(s) + 2 Na+(aq) + 2 NO3-(aq) C) Ca2+(aq) + 2 NO3-(aq) + 2 ...
... The correct complete ionic equation for the reaction that occurs when aqueous solutions of Ca(NO3)2 and Na2CO3 are mixed is A) Ca(NO3)2(aq) + Na2CO3(aq) ----> CaCO3(s) + 2 NaNO3(aq) B) Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + CO32-(aq) ----> CaCO3(s) + 2 Na+(aq) + 2 NO3-(aq) C) Ca2+(aq) + 2 NO3-(aq) + 2 ...
Year End Review
... is a yellow precipitate. How much precipitate would be produced if 6.00 g of sodium iodide was used with sufficient NaI? If hydrogen gas occupies 44. 8 L at STP, at what pressure will the sample occupy 112 L when the temperature is fixed at 30 0C ? What is the volume occupied by 4.4 g carbon dioxide ...
... is a yellow precipitate. How much precipitate would be produced if 6.00 g of sodium iodide was used with sufficient NaI? If hydrogen gas occupies 44. 8 L at STP, at what pressure will the sample occupy 112 L when the temperature is fixed at 30 0C ? What is the volume occupied by 4.4 g carbon dioxide ...
Fall Final 2009
... The balanced net ionic equation for precipitation of PbSO4 when aqueous solutions of Pb(NO3)2 and Na2SO4 are mixed is; (a) Pb+2(aq) + 2 NO3–(aq) + 2Na+(aq) + SO4-2(aq) ------> PbSO4(s) + 2 NO3-1(aq) + 2Na+(aq) (b) Pb+2(aq) + SO4-2(aq) ------> PbSO4(s) (c) Pb+2(aq) + 2 NO3–(aq) ------> Pb(NO3)2 (aq) ...
... The balanced net ionic equation for precipitation of PbSO4 when aqueous solutions of Pb(NO3)2 and Na2SO4 are mixed is; (a) Pb+2(aq) + 2 NO3–(aq) + 2Na+(aq) + SO4-2(aq) ------> PbSO4(s) + 2 NO3-1(aq) + 2Na+(aq) (b) Pb+2(aq) + SO4-2(aq) ------> PbSO4(s) (c) Pb+2(aq) + 2 NO3–(aq) ------> Pb(NO3)2 (aq) ...
Chemistry in Society Homework Booklet
... The following equilibrium involves two compounds of phosphorus. PCl3(g) + 3NH3 (g) P(NH2)3(g) + 3HCl(g) (a) An increase in temperature moves the equilibrium to the left. What does this indicate about the enthalpy change for the forward reaction? (b) What effect, if any, will an increase in pressure ...
... The following equilibrium involves two compounds of phosphorus. PCl3(g) + 3NH3 (g) P(NH2)3(g) + 3HCl(g) (a) An increase in temperature moves the equilibrium to the left. What does this indicate about the enthalpy change for the forward reaction? (b) What effect, if any, will an increase in pressure ...
CHEMISTRY
... the periodic table. Patterns help reduce the amount of things we need to memorize and also allow us to acquire information quickly. For example, knowing that an element is in group 2 tells us a lot about that element. We know it has two valence electrons, it’s a fairly reactive metal and it forms an ...
... the periodic table. Patterns help reduce the amount of things we need to memorize and also allow us to acquire information quickly. For example, knowing that an element is in group 2 tells us a lot about that element. We know it has two valence electrons, it’s a fairly reactive metal and it forms an ...
Carbon Dioxide, Carbonic Acid, and Carbonate Equilibria
... amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. • Henry's law can be put into mathematical terms (at constant temperature) as c = p kH c is the concentration of the solute in mol/L ...
... amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. • Henry's law can be put into mathematical terms (at constant temperature) as c = p kH c is the concentration of the solute in mol/L ...
Mid Term Exam Topics 1-5 solution - OCW
... positive and the mixture will present an azeotrope with minimum boiling temperature 5.- (2 points) Citric acid (AH3) is an organic triprotic acid. The pair [AH2-]/[A3-] is commonly used as a buffer in biochemistry. An enzymatic reaction takes place in 10 mL of a buffered solution with total buffer c ...
... positive and the mixture will present an azeotrope with minimum boiling temperature 5.- (2 points) Citric acid (AH3) is an organic triprotic acid. The pair [AH2-]/[A3-] is commonly used as a buffer in biochemistry. An enzymatic reaction takes place in 10 mL of a buffered solution with total buffer c ...
Name - cloudfront.net
... What is the effect of adding more water to the following equilibrium reaction: CO2 + H2O ↔ H2CO3 In an endothermic reaction at equilibrium, what is the effect of raising the temperature? What happens to a catalyst in a reaction? Is the melting of ice at a temperature above 0 oC: a) endothermic, or b ...
... What is the effect of adding more water to the following equilibrium reaction: CO2 + H2O ↔ H2CO3 In an endothermic reaction at equilibrium, what is the effect of raising the temperature? What happens to a catalyst in a reaction? Is the melting of ice at a temperature above 0 oC: a) endothermic, or b ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.