Chapter 20b - U of L Class Index
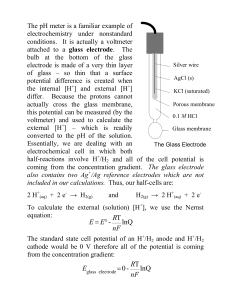
... of glass – so thin that a surface AgCl (s) potential difference is created when the internal [H+] and external [H+] KCl (saturated) differ. Because the protons cannot Porous membrane actually cross the glass membrane, this potential can be measured (by the 0.1 M HCl voltmeter) and used to calculate ...
... of glass – so thin that a surface AgCl (s) potential difference is created when the internal [H+] and external [H+] KCl (saturated) differ. Because the protons cannot Porous membrane actually cross the glass membrane, this potential can be measured (by the 0.1 M HCl voltmeter) and used to calculate ...
1.5.16(Chem) - mrcarlsonschemistryclass
... • Draw the funny way to remember cations and anions: ...
... • Draw the funny way to remember cations and anions: ...
Unit 1
... NaOH Sodium Hydroxide (Drain cleaner) NH4OH Ammonium Hydroxide (Cleaning) NH3 Ammonia (Cleaning) ...
... NaOH Sodium Hydroxide (Drain cleaner) NH4OH Ammonium Hydroxide (Cleaning) NH3 Ammonia (Cleaning) ...
158KB - NZQA
... Whereas HCOOH is a weak acid, it does not readily dissociate in water. HCOOH(aq) + H2O() H3O+(aq) + HCOO–(aq) [H3O+] = 0.00398mol L–1 In the resulting solutions, HCl has a higher concentration of H 3O+, and therefore a lower pH (1) than HCOOH, which has a lower concentration of H3O+, and therefor ...
... Whereas HCOOH is a weak acid, it does not readily dissociate in water. HCOOH(aq) + H2O() H3O+(aq) + HCOO–(aq) [H3O+] = 0.00398mol L–1 In the resulting solutions, HCl has a higher concentration of H 3O+, and therefore a lower pH (1) than HCOOH, which has a lower concentration of H3O+, and therefor ...
Write this into your supplemental packet opposite page
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
SAMPLE EXERCISE 4.5 Comparing Acid Strengths
... Plan: The approach we take is outlined in Table 4.3. We can predict whether a substance is ionic or molecular, based on its composition. As we saw in Section 2.7, most ionic compounds we encounter in this text are composed of a metal and a nonmetal, whereas most molecular compounds are composed only ...
... Plan: The approach we take is outlined in Table 4.3. We can predict whether a substance is ionic or molecular, based on its composition. As we saw in Section 2.7, most ionic compounds we encounter in this text are composed of a metal and a nonmetal, whereas most molecular compounds are composed only ...
First Year - WordPress.com
... dispersion force is the weakest type of intermolecular interactions the strong intermolecular attractions in H2O result from hydrogen bonding boiling point of H2S is less than H2O boiling point of non-polar substances tends to decrease with increasing molecular ...
... dispersion force is the weakest type of intermolecular interactions the strong intermolecular attractions in H2O result from hydrogen bonding boiling point of H2S is less than H2O boiling point of non-polar substances tends to decrease with increasing molecular ...
AP Chemistry Summer Assignment
... 60.A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of su ...
... 60.A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of su ...
Higher Tier, Unit C2: Chemistry
... (c) Temporary hardness in water can be softened by boiling. All hard water can be softened by ion exchange. Explain how both these methods work by setting out clearly what happens and why the water ends up being soft. ...
... (c) Temporary hardness in water can be softened by boiling. All hard water can be softened by ion exchange. Explain how both these methods work by setting out clearly what happens and why the water ends up being soft. ...
OVERVIEW DESCRIPTION DES ADVANTAGES LICENSING
... Lactic acid has been an intermediate-volume specialty chemical for a wide range of food processing and industrial applications that includes pharmaceutical, cosmetics and other chemicals synthesis. Researchers at the South Dakota School of Mines & Technology have developed a unique approach to synth ...
... Lactic acid has been an intermediate-volume specialty chemical for a wide range of food processing and industrial applications that includes pharmaceutical, cosmetics and other chemicals synthesis. Researchers at the South Dakota School of Mines & Technology have developed a unique approach to synth ...
Ionic Bonding
... (e) HCN (f) C2H5OH (g) CH3OCH3 (h) CH3NH2 14. (a) Illustrate the structure of a hydronium ion (H3O+) by drawing its Lewis structure. (b) Name the bonds within the hydronium ion. (c) What kinds of bonds is this ion likely to form with other entities? 15. Is it correct for the structural formula of H2 ...
... (e) HCN (f) C2H5OH (g) CH3OCH3 (h) CH3NH2 14. (a) Illustrate the structure of a hydronium ion (H3O+) by drawing its Lewis structure. (b) Name the bonds within the hydronium ion. (c) What kinds of bonds is this ion likely to form with other entities? 15. Is it correct for the structural formula of H2 ...
Review Packet - Daigneault Chem.is.try
... Organize and review all old exams and reading journals. Study sequentially. Divide your study time into short, intense sections. This can be more effective than studying continually for a long period of time. “Guess the test questions”. You should ask yourself what is most important when stu ...
... Organize and review all old exams and reading journals. Study sequentially. Divide your study time into short, intense sections. This can be more effective than studying continually for a long period of time. “Guess the test questions”. You should ask yourself what is most important when stu ...
Chapter 2 PowerPoint
... In (b), we need to convert the anion to its parent acid shown in Table 2.6. Solution (a) We start with our reference acid, phosphoric acid (H 3PO4). Because H3PO3 has one fewer O atom, it is called phosphorous acid. (b) The parent acid is HIO4. Because the acid has one more O atom than our reference ...
... In (b), we need to convert the anion to its parent acid shown in Table 2.6. Solution (a) We start with our reference acid, phosphoric acid (H 3PO4). Because H3PO3 has one fewer O atom, it is called phosphorous acid. (b) The parent acid is HIO4. Because the acid has one more O atom than our reference ...
Investigating Chemistry - Chemistry at Winthrop University
... • Can you name CaCl2 and PF5 ? They differ. • Note that one is a salt and one is not. • We don’t use prefixes for naming salts (made from a metal plus a nonmetal). • We do use them for molecules. • CaCl2 is just calcium chloride. • But PF5 is phosphorus pentafluoride. ...
... • Can you name CaCl2 and PF5 ? They differ. • Note that one is a salt and one is not. • We don’t use prefixes for naming salts (made from a metal plus a nonmetal). • We do use them for molecules. • CaCl2 is just calcium chloride. • But PF5 is phosphorus pentafluoride. ...
metal-water interactions and hydrogen bond strength
... BaZn(CH3COO)4·2H2O exhibits three bands in the region of the OD vibrations of the matrix-isolated HDO molecules (2568, 2520 and 2334 cm-1, ambient temperature) which shift to lower frequencies on cooling. Furthermore, the band at the lowest wavenumber transforms into two bands at 2282 and 2212 cm-1 ...
... BaZn(CH3COO)4·2H2O exhibits three bands in the region of the OD vibrations of the matrix-isolated HDO molecules (2568, 2520 and 2334 cm-1, ambient temperature) which shift to lower frequencies on cooling. Furthermore, the band at the lowest wavenumber transforms into two bands at 2282 and 2212 cm-1 ...
CHEMISTry is life - World of Teaching
... try every combination possible from the three chemicals that they are given. The reactions can be conducted in sealed plastic baggies. After making some initial observations, students must conduct an experiment of their choice based on the scientific method. ...
... try every combination possible from the three chemicals that they are given. The reactions can be conducted in sealed plastic baggies. After making some initial observations, students must conduct an experiment of their choice based on the scientific method. ...
SUMMER WORK AP Chemistry
... 423 Main Street, Wilbraham, MA 01095-1715 · phone: 413.596.6811 · fax: 413.596.4108 · www.WMA.us ...
... 423 Main Street, Wilbraham, MA 01095-1715 · phone: 413.596.6811 · fax: 413.596.4108 · www.WMA.us ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.