Unit 9: ACIDS AND BASES
... b. State whether a given acid or base is strong or weak. c. Distinguish between strong and weak acids and bases, and determine the relative strengths of acids and bases, using experimental data. The pH Scale a. Distinguish between aqueous solutions that are acidic, neutral or alkaline using the pH s ...
... b. State whether a given acid or base is strong or weak. c. Distinguish between strong and weak acids and bases, and determine the relative strengths of acids and bases, using experimental data. The pH Scale a. Distinguish between aqueous solutions that are acidic, neutral or alkaline using the pH s ...
Chem Reactions (and Balancing Equations)
... •What Happened to the Other Oxygen Atom? •This equation is not balanced! •Two hydrogen atoms from a hydrogen molecule (H2) combines with one of the oxygen atoms from an oxygen molecule (O2) to form H2O. Then, the remaining oxygen atom combines with two more hydrogen atoms (from another H2 molecule) ...
... •What Happened to the Other Oxygen Atom? •This equation is not balanced! •Two hydrogen atoms from a hydrogen molecule (H2) combines with one of the oxygen atoms from an oxygen molecule (O2) to form H2O. Then, the remaining oxygen atom combines with two more hydrogen atoms (from another H2 molecule) ...
111 Exam I Outline
... IV. LIMITING REACTANTS When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the re ...
... IV. LIMITING REACTANTS When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the re ...
Unit 3 Notes
... When a reaction takes place between 2 reactants, it is very unlikely that both of the substances are in exactly the right proportions and that they will both run out at the same time. Usually, one runs out before the other and this reactant limits how much product can be formed. The reaction will be ...
... When a reaction takes place between 2 reactants, it is very unlikely that both of the substances are in exactly the right proportions and that they will both run out at the same time. Usually, one runs out before the other and this reactant limits how much product can be formed. The reaction will be ...
Review Final 111 Lect
... 5. The equilibrium concentration of HSO3- is much higher than the equilibrium conc. of SO32- in the reaction: _ SO3 2- + H3O+ HSO3- + H2O List two conjugate acid /base pairs and label each species as stronger or weaker acid or base. a. b. 6. A. Write the equilibrium equation for the solubility of Ca ...
... 5. The equilibrium concentration of HSO3- is much higher than the equilibrium conc. of SO32- in the reaction: _ SO3 2- + H3O+ HSO3- + H2O List two conjugate acid /base pairs and label each species as stronger or weaker acid or base. a. b. 6. A. Write the equilibrium equation for the solubility of Ca ...
Donnan phenomena in membranes with charge due to ion
... Now consider the interaction between adsorbed charges on neighboring sites. An exact treatment would require the computation of the configurational partition function, for the system of charges covering all possible arrangements on the available sites. This is a formidable problem, and so we elect t ...
... Now consider the interaction between adsorbed charges on neighboring sites. An exact treatment would require the computation of the configurational partition function, for the system of charges covering all possible arrangements on the available sites. This is a formidable problem, and so we elect t ...
I- Introduction
... measurements within the context of the fundamental technological problem with which he is presented. The selection and utilization of suitable chemical procedures requires a wide knowledge of chemistry, whilst familiarity with and the ability to operate a varied range of instruments is essential. Fi ...
... measurements within the context of the fundamental technological problem with which he is presented. The selection and utilization of suitable chemical procedures requires a wide knowledge of chemistry, whilst familiarity with and the ability to operate a varied range of instruments is essential. Fi ...
Topic/Objective - cloudfront.net
... a small test tube and then heated in boiling water until all the liquid vaporizes and fills the tube as excess water escapes. After the gas is cooled, the mass, volume, and pressure is measured to determine the molecular mass of the substance using ideal gas law Title: Determination of the molar vol ...
... a small test tube and then heated in boiling water until all the liquid vaporizes and fills the tube as excess water escapes. After the gas is cooled, the mass, volume, and pressure is measured to determine the molecular mass of the substance using ideal gas law Title: Determination of the molar vol ...
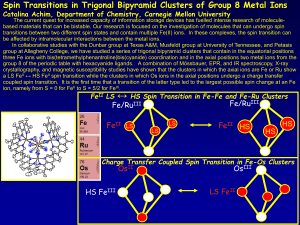
Ch. 20- Electrochemistry
... 5. In general, increasing the concentration of reactants or decreasing the concentration of products increases the driving force for the reaction, resulting in a higher emf. Conversely, decreasing the concentration of reactants or increasing the concentration of products causes the emf to decrease f ...
... 5. In general, increasing the concentration of reactants or decreasing the concentration of products increases the driving force for the reaction, resulting in a higher emf. Conversely, decreasing the concentration of reactants or increasing the concentration of products causes the emf to decrease f ...
Chemical Composition Notes
... _______ - formed when electrons are lost or gained in ordinary chemical reactions; affect size of atoms dramatically __________ - (+) ions; often metals since metals lose electrons to become positively charged ________ - (—) ions; often nonmetals since nonmetals gain electrons to become negatively c ...
... _______ - formed when electrons are lost or gained in ordinary chemical reactions; affect size of atoms dramatically __________ - (+) ions; often metals since metals lose electrons to become positively charged ________ - (—) ions; often nonmetals since nonmetals gain electrons to become negatively c ...
problems - chem.msu.su
... substances are present in the solution, a sharp rise in current occurs at the certain potential. The recorded plot of current against applied voltage I = f(E) (polarogram) is characterized by wave height h (cm), or diffusion current Imax (A), that are proportional to the concentration of the unknown ...
... substances are present in the solution, a sharp rise in current occurs at the certain potential. The recorded plot of current against applied voltage I = f(E) (polarogram) is characterized by wave height h (cm), or diffusion current Imax (A), that are proportional to the concentration of the unknown ...
File
... _______20. Magnesium sulfate, MgSO4 has a molar mass of 120.4 grams. However, it is commonly found in a hydrate called epsom salt, with the formula MgSO4•7H2O. The % of water by mass in this hydrate is closest to A) 1.04% B) 96 % C) 51 % D) 73 % E) 86 % _______21. How many grams of NaOH must be diss ...
... _______20. Magnesium sulfate, MgSO4 has a molar mass of 120.4 grams. However, it is commonly found in a hydrate called epsom salt, with the formula MgSO4•7H2O. The % of water by mass in this hydrate is closest to A) 1.04% B) 96 % C) 51 % D) 73 % E) 86 % _______21. How many grams of NaOH must be diss ...
Review for second exam:
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
intermediate chemistry may 2011 marking scheme
... species (1); thus N in nitrous acid oxidised to +4 in NO2 and reduced to +2 in NO (2 marks) 6. Give the number of: (a) atoms in 8g He 8/4 = 2 mol = 12 X 1023 ...
... species (1); thus N in nitrous acid oxidised to +4 in NO2 and reduced to +2 in NO (2 marks) 6. Give the number of: (a) atoms in 8g He 8/4 = 2 mol = 12 X 1023 ...
2017 Chemistry Exam Review Compounds and Reactions 1. Know
... 37. Define solution in terms of solute and solvent? What is the solvent in an aqueous solution? 38. Describe and show (with electronegativity values and an electron dot diagram) the polarity of a water molecule. 39. Draw water molecules to show how, based on their polarity, they cling together. What ...
... 37. Define solution in terms of solute and solvent? What is the solvent in an aqueous solution? 38. Describe and show (with electronegativity values and an electron dot diagram) the polarity of a water molecule. 39. Draw water molecules to show how, based on their polarity, they cling together. What ...
Example 1-2
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.