Buffer Solutions
... 3. A buffer solution was prepareed by dissolving 2.5 grams of NH4Cl in 125 mL of 0.24 M ammonia solution. At what pH will this solution serve as a buffer? 4. a) A formic acid, sodium formate solution is made up by dissolving 0.2 mole of formic acid and 0.3 mole of sodium formate in 500 mL of water? ...
... 3. A buffer solution was prepareed by dissolving 2.5 grams of NH4Cl in 125 mL of 0.24 M ammonia solution. At what pH will this solution serve as a buffer? 4. a) A formic acid, sodium formate solution is made up by dissolving 0.2 mole of formic acid and 0.3 mole of sodium formate in 500 mL of water? ...
FINAL EXAM REVIEW PROBLEMS
... 52. Calculate the molarity of a solution prepared by dissolving 2.75 g of ethanol, C2H5OH, in enough water to give a final volume of 101 mL. 53. Give the concentrations of the ions in each of the following solutions: a. 0.10 M Na2CO3 b. 0.010 M Al2(SO4)3 54. Calculate the number of moles of Cl- ions ...
... 52. Calculate the molarity of a solution prepared by dissolving 2.75 g of ethanol, C2H5OH, in enough water to give a final volume of 101 mL. 53. Give the concentrations of the ions in each of the following solutions: a. 0.10 M Na2CO3 b. 0.010 M Al2(SO4)3 54. Calculate the number of moles of Cl- ions ...
CHEM 32-002
... f) Te6_____ 9) Alkenes react with water very slowly. However, this reaction goes much faster if H2SO4 is added. In terms of energy, the H2SO4... a) makes the activation energy larger b) makes the activation energy smaller c) makes the heat of reaction (H) larger d) makes the heat of reaction smalle ...
... f) Te6_____ 9) Alkenes react with water very slowly. However, this reaction goes much faster if H2SO4 is added. In terms of energy, the H2SO4... a) makes the activation energy larger b) makes the activation energy smaller c) makes the heat of reaction (H) larger d) makes the heat of reaction smalle ...
equilibrium and activation energy
... What direction does the equilibrium shift if more oxygen is added? What direction does the equilibrium shift if water is removed? How does the concentration of methanol (CH3OH) change if more oxygen is added? How does the concentration of methanol change if more water is added? How does the concentr ...
... What direction does the equilibrium shift if more oxygen is added? What direction does the equilibrium shift if water is removed? How does the concentration of methanol (CH3OH) change if more oxygen is added? How does the concentration of methanol change if more water is added? How does the concentr ...
FORMULA WRITNG
... 7) Write the electron dot structures (Lewis dot structures) for the compounds made from the following elements: (Be sure to write the structures for compounds according to whether they are ionic or covalent.) a. Rb and O b. Mg and S c. C and F d. Se and F ...
... 7) Write the electron dot structures (Lewis dot structures) for the compounds made from the following elements: (Be sure to write the structures for compounds according to whether they are ionic or covalent.) a. Rb and O b. Mg and S c. C and F d. Se and F ...
The Electrical Double Layer and Its Structure
... rigid Helmholtz layer and the diffuse one of Gouy and Chapman. Specific adsorption of ions at the metal surface was pointed out by Graham in 1947. In consecutive developments, the role of the solvent has been taken into account (Parsons 1954; Bockris 1963). It soon became clear that in dipolar solve ...
... rigid Helmholtz layer and the diffuse one of Gouy and Chapman. Specific adsorption of ions at the metal surface was pointed out by Graham in 1947. In consecutive developments, the role of the solvent has been taken into account (Parsons 1954; Bockris 1963). It soon became clear that in dipolar solve ...
Measurements and Mathematics in Physics
... agreement when the same quantity in measured several times ...
... agreement when the same quantity in measured several times ...
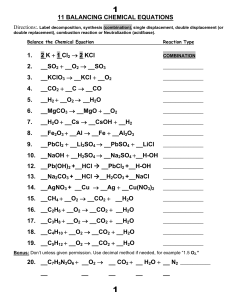
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... The missing product in the reaction:__HCl +__ Ba(OH)2 Æ _ ___ + _ BaCl2 is: [H‐OH HBa Cl‐OH] ...
... The missing product in the reaction:__HCl +__ Ba(OH)2 Æ _ ___ + _ BaCl2 is: [H‐OH HBa Cl‐OH] ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... c. some MATTER exists in elemental form [(e.g.) gold [Au] = not chemically REACTIVE)] *d. ELEMENTS individually or combined form everything in the universe including HUMANS *1. Human body’s most abundant ELEMENTS: carbon [C], oxygen [O], hydrogen [H], and nitrogen [N]; for teeth & BONES = calcium [C ...
... c. some MATTER exists in elemental form [(e.g.) gold [Au] = not chemically REACTIVE)] *d. ELEMENTS individually or combined form everything in the universe including HUMANS *1. Human body’s most abundant ELEMENTS: carbon [C], oxygen [O], hydrogen [H], and nitrogen [N]; for teeth & BONES = calcium [C ...
Section 8-2
... • Exception to the prefixes in table 8.3: – The first element never uses the mono prefix – If using a prefix would cause two consecutive vowels, one of the them is often dropped to avoid a difficult pronunciation. • CO is monoxide NOT monooxide. ...
... • Exception to the prefixes in table 8.3: – The first element never uses the mono prefix – If using a prefix would cause two consecutive vowels, one of the them is often dropped to avoid a difficult pronunciation. • CO is monoxide NOT monooxide. ...
Balancing Chemical Equations
... Vocabulary: coefficient, compound, decomposition, double replacement, element, molecule, product, reactant, single replacement, subscript, synthesis ...
... Vocabulary: coefficient, compound, decomposition, double replacement, element, molecule, product, reactant, single replacement, subscript, synthesis ...
Chemistry (Theory)
... When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation. If both of them contain α-hydrogen atoms, it gives a mixture of four products. ...
... When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation. If both of them contain α-hydrogen atoms, it gives a mixture of four products. ...
Lectures 28-31 - U of L Class Index
... The maximum work that can be done on the system is rG. Since rG < 0 for a spontaneous reaction, a spontaneous reaction can do work on the surroundings. The maximum amount of non-PV work that can be done on the surroundings is equal to -rG! ...
... The maximum work that can be done on the system is rG. Since rG < 0 for a spontaneous reaction, a spontaneous reaction can do work on the surroundings. The maximum amount of non-PV work that can be done on the surroundings is equal to -rG! ...
Review for Physical Science Test #2
... To tell a strong acid from a weak acid To tell an acid from a neutral solution To tell a strong base from a weak base To create a temporary tattoo on your little sister’s face right before picture day. ...
... To tell a strong acid from a weak acid To tell an acid from a neutral solution To tell a strong base from a weak base To create a temporary tattoo on your little sister’s face right before picture day. ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.