Chemistry Review 2 answer key
... State evidence from the balanced equation for the cell with iron and copper electrodes that indicates the reaction in the cell is an oxidation-reduction reaction. [1] In an oxidation-reduction reaction, changes in oxidation states are observed. In this reaction, Cu changes from Cu2+ to Cu0, and Fe c ...
... State evidence from the balanced equation for the cell with iron and copper electrodes that indicates the reaction in the cell is an oxidation-reduction reaction. [1] In an oxidation-reduction reaction, changes in oxidation states are observed. In this reaction, Cu changes from Cu2+ to Cu0, and Fe c ...
Solutions for Chapter 8 End-of-Chapter Problems
... of dirty socks should equal 1, given that the person doing the washing is careful and looks for all the socks that may be hidden in other clothes or those that are dropped somewhere in the process. However, in the experience of this author, the probability hovers mysteriously around ...
... of dirty socks should equal 1, given that the person doing the washing is careful and looks for all the socks that may be hidden in other clothes or those that are dropped somewhere in the process. However, in the experience of this author, the probability hovers mysteriously around ...
Reliable Computation of Equilibrium States and Bifurcations in Food
... solutions, and so the total number of equilibrium states may be unknown a priori. For simple models, it may be possible to solve for many of the equilibrium states analytically, but for more complex models a computational method is needed that is capable of finding, with certainty, all the solutions ...
... solutions, and so the total number of equilibrium states may be unknown a priori. For simple models, it may be possible to solve for many of the equilibrium states analytically, but for more complex models a computational method is needed that is capable of finding, with certainty, all the solutions ...
chemistry - Textbooks Online
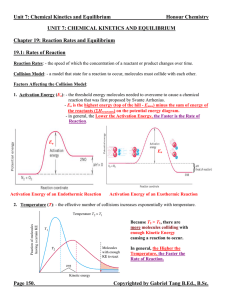
... integral values. For an elementary reaction, its order is never fractional since it is a one step process. (ii) Order of a reaction should be determined only by experiments. It cannot be predicted interms of stoichiometry of reactants and products. (iii)Simple reactions possess low values of order l ...
... integral values. For an elementary reaction, its order is never fractional since it is a one step process. (ii) Order of a reaction should be determined only by experiments. It cannot be predicted interms of stoichiometry of reactants and products. (iii)Simple reactions possess low values of order l ...
CHAPTER 21 ELECTROCHEMISTRY: CHEMICAL CHANGE AND
... Balance oxidation half-reaction: balance O Mn2+(aq) + 4 H2O(l) → MnO4−(aq) Mn2+(aq) + 4 H2O(l) → MnO4−(aq) + 8 H+(aq) balance H Mn2+(aq) + 4 H2O(l) → MnO4−(aq) + 8 H+(aq) + 5 ebalance H Multiply reduction half-reaction by 2 and oxidation half-reaction by 2 to transfer 10 e- in the overall reaction. ...
... Balance oxidation half-reaction: balance O Mn2+(aq) + 4 H2O(l) → MnO4−(aq) Mn2+(aq) + 4 H2O(l) → MnO4−(aq) + 8 H+(aq) balance H Mn2+(aq) + 4 H2O(l) → MnO4−(aq) + 8 H+(aq) + 5 ebalance H Multiply reduction half-reaction by 2 and oxidation half-reaction by 2 to transfer 10 e- in the overall reaction. ...
CHAPTER 4 REACTIONS IN AQUEOUS SOLUTIONS
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
Thermal Decomposition of the Non-Interstitial Hydrides for the
... many advances in hydrogen production8 and utilization technologies have been made during the past decade. However, there remain a number of fundamental scientific, technological, and socio-economic problems to be overcome before any large-scale utilization of hydrogen in our day-to-day human activit ...
... many advances in hydrogen production8 and utilization technologies have been made during the past decade. However, there remain a number of fundamental scientific, technological, and socio-economic problems to be overcome before any large-scale utilization of hydrogen in our day-to-day human activit ...
chemistry - Ethiopian Ministry of Education
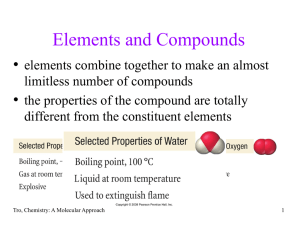
... The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated by distance. Since chemistry is ...
... The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated by distance. Since chemistry is ...
8. Solution Guide to Supplementary Exercises
... According to the equation of Reaction 2, 1 mole of HCO3–(aq) would react with 1 mole of H+(aq). i.e. 0.0800 mole of HCO3–(aq) would react with 0.0800 mole of H+(aq). Hence H+(aq) was in excess. HCO3–(aq) was the limiting reactant. +334 J 0.0800 mol = +4 180 J mol–1 = +4.18 kJ mol–1 ...
... According to the equation of Reaction 2, 1 mole of HCO3–(aq) would react with 1 mole of H+(aq). i.e. 0.0800 mole of HCO3–(aq) would react with 0.0800 mole of H+(aq). Hence H+(aq) was in excess. HCO3–(aq) was the limiting reactant. +334 J 0.0800 mol = +4 180 J mol–1 = +4.18 kJ mol–1 ...
Chapter 6 Thermodynamics: The First Law
... For the two processes, the initial and final states are the same, so ∆U is also the same. In the first process, mechanical work is done on the system, q = 0, and ∆U = w. In the second process, w = 0, and ∆U = q. Both q and w are clearly path dependent. Note: ...
... For the two processes, the initial and final states are the same, so ∆U is also the same. In the first process, mechanical work is done on the system, q = 0, and ∆U = w. In the second process, w = 0, and ∆U = q. Both q and w are clearly path dependent. Note: ...
Calculations with Chemical Formulas and Equations
... analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been Stoichiometry ...
... analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been Stoichiometry ...
Molecular-level mechanisms of quartz dissolution under neutral and
... 2005). While the dissolution of most silicate minerals is promoted largely by the H+ ion, the dissolution of quartz is decreased slightly upon adding H+ ions until a very acidic condition is attained (i.e., pH = 2) (Dove and Elston, 1992; Nangia and Garrison, 2008, 2009). A thin amorphous layer has ...
... 2005). While the dissolution of most silicate minerals is promoted largely by the H+ ion, the dissolution of quartz is decreased slightly upon adding H+ ions until a very acidic condition is attained (i.e., pH = 2) (Dove and Elston, 1992; Nangia and Garrison, 2008, 2009). A thin amorphous layer has ...
SCH3U0FinalExamReview - Savita Pall and Chemistry
... (d) O, O2-, S, S210. Which ion would be larger: (a) Fe2+ or Fe3+, (b) O- or O2-? 11. What two factors are most important in determining the size of an atom? 12. Explain the relative sizes of the atoms within a given group of the periodic table. Illustrate your answer with specific examples. 13. Comp ...
... (d) O, O2-, S, S210. Which ion would be larger: (a) Fe2+ or Fe3+, (b) O- or O2-? 11. What two factors are most important in determining the size of an atom? 12. Explain the relative sizes of the atoms within a given group of the periodic table. Illustrate your answer with specific examples. 13. Comp ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... though this fire does not have much in common with a coal-burning power plant. Both the fire and the power plant, however, are technologies that harness energy-producing processes. Humans continually devise new technologies that use chemical reactions to produce materials with useful properties. Sin ...
... though this fire does not have much in common with a coal-burning power plant. Both the fire and the power plant, however, are technologies that harness energy-producing processes. Humans continually devise new technologies that use chemical reactions to produce materials with useful properties. Sin ...
SUPPORTING INFORMATION Obtaining equation 8 Consider a
... applied to other receptors, such as surfactant monomers and micelles, for example. In the absence of a quencher, the intensity of emission of the fluorophore, in the presence of two cyclodextrins, CD1 and CD2, would be: ...
... applied to other receptors, such as surfactant monomers and micelles, for example. In the absence of a quencher, the intensity of emission of the fluorophore, in the presence of two cyclodextrins, CD1 and CD2, would be: ...
Water Chemistry - U
... questions: (1) is there a need for another text in the field, and (2) how will their text be different from what is already available? It is obvious from the fact that this book exists that we answered yes to the first question. Our reasons for doing so are based on our answers to the second question, ...
... questions: (1) is there a need for another text in the field, and (2) how will their text be different from what is already available? It is obvious from the fact that this book exists that we answered yes to the first question. Our reasons for doing so are based on our answers to the second question, ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.