Document
... theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry (using LR only) 1 mol Al 3 mol Cu 0.030 g Al x x = 0.0017 mol Cu 26.98 g Al 2 mol Al • This is different from the actual yield, the amount one actually produces and measures (or experimental) ...
... theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry (using LR only) 1 mol Al 3 mol Cu 0.030 g Al x x = 0.0017 mol Cu 26.98 g Al 2 mol Al • This is different from the actual yield, the amount one actually produces and measures (or experimental) ...
1ST CHAPTER Long-questions-basic-concept
... with electrons. The force of collision knocked out electrons from atoms. Usually, one electron is removed form an atom. The atoms turn into positive ions. These positive ions have different masses depending upon the nature of the isotopes present in them. The positive ion of each isotope has its own ...
... with electrons. The force of collision knocked out electrons from atoms. Usually, one electron is removed form an atom. The atoms turn into positive ions. These positive ions have different masses depending upon the nature of the isotopes present in them. The positive ion of each isotope has its own ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: C. The ideal gas equation can be used to calculate the number of moles of gas needed to achieve a specific gas volume (the desired volume of the airbag) at specific pressure and temperature conditions. A is the Arrhenius equation and can be used to calculate the rate constant or ac ...
... Correct Response: C. The ideal gas equation can be used to calculate the number of moles of gas needed to achieve a specific gas volume (the desired volume of the airbag) at specific pressure and temperature conditions. A is the Arrhenius equation and can be used to calculate the rate constant or ac ...
mcdonald (pam78654) – HW 1: High School Concepts – laude
... mcdonald (pam78654) – HW 1: High School Concepts – laude – (89560) This print-out should have 40 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. 001 10.0 points Calculate the number of H2 O molecules in 1.00 cm3 of water at 0◦ C (dens ...
... mcdonald (pam78654) – HW 1: High School Concepts – laude – (89560) This print-out should have 40 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. 001 10.0 points Calculate the number of H2 O molecules in 1.00 cm3 of water at 0◦ C (dens ...
Chem 33 Lab - Santa Clara University
... Company (available from the bookstore); you may use the same notebook as you purchased for Chem32L 4. Safety splash goggles 5. Laboratory coat (available from the bookstore) LABORATORY GUIDELINES 1. The laboratory in Daly Science 100 were modernized and redesigned in 1994, with safety as the primary ...
... Company (available from the bookstore); you may use the same notebook as you purchased for Chem32L 4. Safety splash goggles 5. Laboratory coat (available from the bookstore) LABORATORY GUIDELINES 1. The laboratory in Daly Science 100 were modernized and redesigned in 1994, with safety as the primary ...
c00kieee - Ritter Illustration
... α-particle causes the radiolysis of water, producing H and OH radicals as well as hydrogen peroxide. In acidic conditions, these species reduce Pu4+ and PuO2 2+ ions to give Pu3+ and PuO2 + , respectively. The radiolysis along with the disproportionation and reproportionation reactions shown in Sche ...
... α-particle causes the radiolysis of water, producing H and OH radicals as well as hydrogen peroxide. In acidic conditions, these species reduce Pu4+ and PuO2 2+ ions to give Pu3+ and PuO2 + , respectively. The radiolysis along with the disproportionation and reproportionation reactions shown in Sche ...
organic problems - St. Olaf College
... 25 Which of the following molecular formulas is reasonable for a stable compound? A) C8H14O2Cl B) C6H14Br2 C) C7H10NF D) C30H54N2Cl 26 What formal charges are present in the molecule C6H5C≡N-O? ( all heavy atoms have a valence shell octet, and C6H5- is a phenyl group) A) N is -1 and C is +1 B) N is ...
... 25 Which of the following molecular formulas is reasonable for a stable compound? A) C8H14O2Cl B) C6H14Br2 C) C7H10NF D) C30H54N2Cl 26 What formal charges are present in the molecule C6H5C≡N-O? ( all heavy atoms have a valence shell octet, and C6H5- is a phenyl group) A) N is -1 and C is +1 B) N is ...
Sample pages 2 PDF
... Actually, these structures can co-exist during synthesis of one or other nanoparticle. The essence of the problem is fine regulation of growth of seed populations for producing metal nanoparticles with desired shape and morphology. The possibilities for its realization are provided by thermodynamic ...
... Actually, these structures can co-exist during synthesis of one or other nanoparticle. The essence of the problem is fine regulation of growth of seed populations for producing metal nanoparticles with desired shape and morphology. The possibilities for its realization are provided by thermodynamic ...
Nanoporous Materials for Hydrogen Storage and H2/D2 Isotope
... each other. Recently, quantum sieving in confined space has received increased attention as an efficient method for hydrogen isotope separation. Despite many theoretical calculations, however, it has been difficult to identify a feasible microporous material up to now. Among various porous materials ...
... each other. Recently, quantum sieving in confined space has received increased attention as an efficient method for hydrogen isotope separation. Despite many theoretical calculations, however, it has been difficult to identify a feasible microporous material up to now. Among various porous materials ...
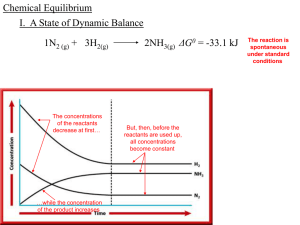
Chapter 15: Chemical Equilibrium
... chemical reactions that is the basis of chemical equilibrium. Reversibility is a central and important aspect of chemical activity in many areas of nature and commerce. For example, ...
... chemical reactions that is the basis of chemical equilibrium. Reversibility is a central and important aspect of chemical activity in many areas of nature and commerce. For example, ...
Chapter 15
... chemical reactions that is the basis of chemical equilibrium. Reversibility is a central and important aspect of chemical activity in many areas of nature and commerce. For example, ...
... chemical reactions that is the basis of chemical equilibrium. Reversibility is a central and important aspect of chemical activity in many areas of nature and commerce. For example, ...
Specification and sample assessment material - Edexcel
... Group 7 elements – chlorine, bromine and iodine ...
... Group 7 elements – chlorine, bromine and iodine ...
Flotation behaviour and surface characteristics of the artificial
... (Zhang et al., 2011). Therefore, the upgrading of titanium slag is an urgent need in the titanium industry. Flotation can be implemented to upgrade titanium slag, as it has long served the mineral processing field as a mature technology (Bahri et al., 2016; Albrecht et al., 2016). The primary titani ...
... (Zhang et al., 2011). Therefore, the upgrading of titanium slag is an urgent need in the titanium industry. Flotation can be implemented to upgrade titanium slag, as it has long served the mineral processing field as a mature technology (Bahri et al., 2016; Albrecht et al., 2016). The primary titani ...
introduction - TestBankTop
... Strategy: For subtraction and addition, the number of significant figures to the right of the decimal point in that part of the calculation is determined by the lowest number of digits to the right of the decimal point in any of the original numbers. For the division part of the calculation, the num ...
... Strategy: For subtraction and addition, the number of significant figures to the right of the decimal point in that part of the calculation is determined by the lowest number of digits to the right of the decimal point in any of the original numbers. For the division part of the calculation, the num ...
RIKEN Accelerator Progress Report
... nuclear physics, atomic and solid state physics, radiochemistry, material engineering, radiation chemistry and ...
... nuclear physics, atomic and solid state physics, radiochemistry, material engineering, radiation chemistry and ...
Section 1.3 - The Student Room
... a Standard enthalpy change of combustion is the enthalpy change when 1 mole of the compound is burnt completely in oxygen, under standard conditions (ie the compound and the products in their most stable states at 1 atmosphere pressure and at a stated temperature, often 298 K). b Standard enthalpy c ...
... a Standard enthalpy change of combustion is the enthalpy change when 1 mole of the compound is burnt completely in oxygen, under standard conditions (ie the compound and the products in their most stable states at 1 atmosphere pressure and at a stated temperature, often 298 K). b Standard enthalpy c ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.