RedOx notes:
... half reactions will allow you to balance some equations that you would find difficult otherwise. Oxidation numbers help us keep track of the movement of electrons (what chemistry is really all about) We need to learn how to assign oxidation numbers ...
... half reactions will allow you to balance some equations that you would find difficult otherwise. Oxidation numbers help us keep track of the movement of electrons (what chemistry is really all about) We need to learn how to assign oxidation numbers ...
Physical Sciences Grade 12 Term 2
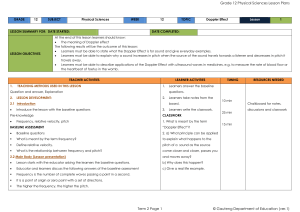
... travelling in his car, hears the siren of the alarm of a house and approaches the house at 2.1 Name the phenomenon that explains the change in the observed frequency. 2.2 Calculate the speed at which the patrol car approaches the house. Use the speed of sound in air as 340 m•s-1. 2.3 If the patrol c ...
... travelling in his car, hears the siren of the alarm of a house and approaches the house at 2.1 Name the phenomenon that explains the change in the observed frequency. 2.2 Calculate the speed at which the patrol car approaches the house. Use the speed of sound in air as 340 m•s-1. 2.3 If the patrol c ...
INTRODUCTION - Test Bank wizard
... Student A: 47.7 mL Student B: 47.1 mL Student C: 47.8 mL From these calculations, we can conclude that the volume measurements made by student B were the most accurate of the three students. The precision in the measurements made by both students B and C are fairly high, although the measurements by ...
... Student A: 47.7 mL Student B: 47.1 mL Student C: 47.8 mL From these calculations, we can conclude that the volume measurements made by student B were the most accurate of the three students. The precision in the measurements made by both students B and C are fairly high, although the measurements by ...
Stoichiometry: Calculations with Chemical Formulas and
... When 0.255 g of caproic acid is burned, you get 0.512 g CO2 and 0.209 g H2O. What is the emp formula of the acid? #mole CO2 = mass/GFM = 0.512g / 44.0g/mol = 0.0116 mol 0.0116 mol CO2 * (1 mole C/1 mol CO2) = 0.0116 mol C 0.0116 mol C * (12.0 g/mol) = 0.140 g C #mole H2O = mass/GFM = 0.209g / 18.0g/ ...
... When 0.255 g of caproic acid is burned, you get 0.512 g CO2 and 0.209 g H2O. What is the emp formula of the acid? #mole CO2 = mass/GFM = 0.512g / 44.0g/mol = 0.0116 mol 0.0116 mol CO2 * (1 mole C/1 mol CO2) = 0.0116 mol C 0.0116 mol C * (12.0 g/mol) = 0.140 g C #mole H2O = mass/GFM = 0.209g / 18.0g/ ...
Version 1.6 - Clark Science Center
... the structure, color, and reactivity of molecules. Structure means we want to understand the arrangement in space of the nuclei and learn what we can about where the electrons are to be found between those nuclei. Also, how those structures influence the chemistry of the materials. Color is of inter ...
... the structure, color, and reactivity of molecules. Structure means we want to understand the arrangement in space of the nuclei and learn what we can about where the electrons are to be found between those nuclei. Also, how those structures influence the chemistry of the materials. Color is of inter ...
Organic Chemistry Organic Chemistry
... We will begin our study of organic families with a review of hydrocarbons, many of which contain multiple bonds between carbon atoms, a functional group with characteristic properties. Fossil fuels (Figure 1) contain mainly hydrocarbons: simple molecules of hydrogen and carbon that are the result of ...
... We will begin our study of organic families with a review of hydrocarbons, many of which contain multiple bonds between carbon atoms, a functional group with characteristic properties. Fossil fuels (Figure 1) contain mainly hydrocarbons: simple molecules of hydrogen and carbon that are the result of ...
Chapter 12
... that four atoms of iron react with three molecules of oxygen to produce two formula units of iron(III) oxide. But, remember that coefficients in an equation represent not only numbers of individual particles but also numbers of moles of particles. Therefore, you can also say that four moles of iron ...
... that four atoms of iron react with three molecules of oxygen to produce two formula units of iron(III) oxide. But, remember that coefficients in an equation represent not only numbers of individual particles but also numbers of moles of particles. Therefore, you can also say that four moles of iron ...
Chemical Equilibria - Beck-Shop
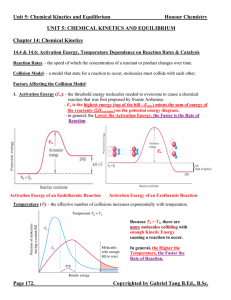
... A: From a kinetics perspective, Qc < Kc implies that the concentrations of the reactant particles are greater than the product particles as compared to if the system is at equilibrium. Thus, we expect a higher effective collision frequency for the forward reaction than that for the backward reaction. ...
... A: From a kinetics perspective, Qc < Kc implies that the concentrations of the reactant particles are greater than the product particles as compared to if the system is at equilibrium. Thus, we expect a higher effective collision frequency for the forward reaction than that for the backward reaction. ...
Thermodynamics - Shailendra Kumar Chemistry
... For a particular chemical reaction, ∆H° = +60.0 kJ and ∆S° = +121 J/K. At what temperature (in K) would this reaction become spontaneous? ...
... For a particular chemical reaction, ∆H° = +60.0 kJ and ∆S° = +121 J/K. At what temperature (in K) would this reaction become spontaneous? ...
1 1411_chapter 6 exercises with answers CHEM 1411, chapter 6
... 43. A glass containing 200. g of H2O at 20°C was placed in a refrigerator. The water loses 11.7 kJ as it cools to a constant temperature. What is its new temperature? The specific heat of water is 4.184 J/g·°C. A) 0.013°C B) 4°C C) 6°C D) 14°C E) 34°C 44. The enthalpy change when a strong acid is n ...
... 43. A glass containing 200. g of H2O at 20°C was placed in a refrigerator. The water loses 11.7 kJ as it cools to a constant temperature. What is its new temperature? The specific heat of water is 4.184 J/g·°C. A) 0.013°C B) 4°C C) 6°C D) 14°C E) 34°C 44. The enthalpy change when a strong acid is n ...
SCH3U: Final Exam Review Note: These questions a
... 43. 65 mL of a 2.5 mol/L solution of silver nitrate is added to an excess of calcium chloride. Identify the precipitate, and calculate the mass of this precipitate that is formed. 44. An excess of sodium carbonate solution is added to 75.0 mL of calcium chloride solution. 7.50 g of precipitate is fo ...
... 43. 65 mL of a 2.5 mol/L solution of silver nitrate is added to an excess of calcium chloride. Identify the precipitate, and calculate the mass of this precipitate that is formed. 44. An excess of sodium carbonate solution is added to 75.0 mL of calcium chloride solution. 7.50 g of precipitate is fo ...
No Slide Title
... empirical formula mass: 14.01+2 (16.00) = 46.01 g/mol n = molar mass = 92.0 g/mol emp. f. mass 46.01 g/mol n = 2 So the molecular formula is twice the size of the empirical formula NO2 = N2O4 ...
... empirical formula mass: 14.01+2 (16.00) = 46.01 g/mol n = molar mass = 92.0 g/mol emp. f. mass 46.01 g/mol n = 2 So the molecular formula is twice the size of the empirical formula NO2 = N2O4 ...
IB Chemistry Online SAQ_Ans
... However, the electron affinities of nitrogen and phosphorus are relatively low. This is due to the presence of half-filled p sub-shells which increases inter-electron repulsion and decreases electron affinity. ...
... However, the electron affinities of nitrogen and phosphorus are relatively low. This is due to the presence of half-filled p sub-shells which increases inter-electron repulsion and decreases electron affinity. ...
Unit 1 - Physical Chemistry REACTION KINETICS
... Relatively large volumes of each reactant are mixed and then small portions (aliquots) of the reacting mixture are removed (pipetted) at regular intervals. These aliquots are usually added to another reagent, which immediately stops or quenches the reaction. Sometimes the aliquot is pipetted into a ...
... Relatively large volumes of each reactant are mixed and then small portions (aliquots) of the reacting mixture are removed (pipetted) at regular intervals. These aliquots are usually added to another reagent, which immediately stops or quenches the reaction. Sometimes the aliquot is pipetted into a ...
The Mole
... What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? 25 mol O2, 50 mol H2O ...
... What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? 25 mol O2, 50 mol H2O ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.