activity series
... Formulas show chemistry at a standstill. Equations show chemistry in action! A. Equations show: ...
... Formulas show chemistry at a standstill. Equations show chemistry in action! A. Equations show: ...
Fall.2008.Week9.Lesson.1 - reich
... (g) means the substance is a gas (l) means the substance is a liquid (s) means the substance is a solid (aq) means the substance is aqueous Aqueous means dissolved in water, which does not necessarily mean the compound was a liquid. Ethanol and sugar both become aqueous, but only one of them was a s ...
... (g) means the substance is a gas (l) means the substance is a liquid (s) means the substance is a solid (aq) means the substance is aqueous Aqueous means dissolved in water, which does not necessarily mean the compound was a liquid. Ethanol and sugar both become aqueous, but only one of them was a s ...
Chapter 11 Chemical Reactions
... If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just ...
... If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just ...
Document
... molecules and small numbers after certain atoms within a molecule. The little number is called the subscript and tells how many of a certain type of atom are in a molecule. The bigger number is called the coefficient and tells how many of a particular type of molecule there are. If there is a coeffi ...
... molecules and small numbers after certain atoms within a molecule. The little number is called the subscript and tells how many of a certain type of atom are in a molecule. The bigger number is called the coefficient and tells how many of a particular type of molecule there are. If there is a coeffi ...
2 - My CCSD
... Law of Conservation of Mass Can only be balanced by changing the coefficients. Has special symbols to indicate the physical state, if a catalyst or energy is required, etc. ...
... Law of Conservation of Mass Can only be balanced by changing the coefficients. Has special symbols to indicate the physical state, if a catalyst or energy is required, etc. ...
Document
... Complete the table that shows the reaction, if any, of the oxides with acid and alkali. Indicate a reaction with "R" and no reaction with "NR". ...
... Complete the table that shows the reaction, if any, of the oxides with acid and alkali. Indicate a reaction with "R" and no reaction with "NR". ...
Topic 16 Some non-metals and their compounds notes
... Some changes to farming practice could reduce these problems. Farmers could, for example: not apply fertilisers when rain is expected, so that they are not washed into water courses before the plants have had a chance to absorb them apply fertilisers several times in small doses rather than in f ...
... Some changes to farming practice could reduce these problems. Farmers could, for example: not apply fertilisers when rain is expected, so that they are not washed into water courses before the plants have had a chance to absorb them apply fertilisers several times in small doses rather than in f ...
File
... Name: _______________________________________________________________________ Period: ____ 11.2: Types of Chemical Reactions Part A: Completion Directions: Each blank can be completed with a term, short phrase, or number. It is possible to __1__ the products of some chemical ...
... Name: _______________________________________________________________________ Period: ____ 11.2: Types of Chemical Reactions Part A: Completion Directions: Each blank can be completed with a term, short phrase, or number. It is possible to __1__ the products of some chemical ...
Chemical Reactions: Introduction to Reaction Types
... solid, (s). For a precipitation reaction to occur, at least one of the products must be insoluble; if both products are soluble, then no reaction occurs. The presence of a precipitate is observed in the lab as a cloudy mixture that results when two solutions are mixed. The following is an example of ...
... solid, (s). For a precipitation reaction to occur, at least one of the products must be insoluble; if both products are soluble, then no reaction occurs. The presence of a precipitate is observed in the lab as a cloudy mixture that results when two solutions are mixed. The following is an example of ...
students - Teach-n-Learn-Chem
... A reaction has occurred if the chemical and physical properties of the reactants and products differ. ...
... A reaction has occurred if the chemical and physical properties of the reactants and products differ. ...
Unit 1: Building Blocks Homework
... Zinc reacts with dilute hydrochloric acid producing hydrogen gas. ...
... Zinc reacts with dilute hydrochloric acid producing hydrogen gas. ...
03. The Theoretic bases of bioenergetics
... 2.Н(formation)= ΣnНf298(products) - ΣnНf298(reactants) 3.Н(combustion) = ΣnНс298(reactants) - ΣnНс298(products) ...
... 2.Н(formation)= ΣnНf298(products) - ΣnНf298(reactants) 3.Н(combustion) = ΣnНс298(reactants) - ΣnНс298(products) ...
~The different types of gases~ Oxygen (O2) The most common
... sustain life. They are inert gases which do not react. These gases can only be combined with other chemical substances with great difficulty. This extreme inertness quality in these gases makes them very valuable for certain applications. ...
... sustain life. They are inert gases which do not react. These gases can only be combined with other chemical substances with great difficulty. This extreme inertness quality in these gases makes them very valuable for certain applications. ...
CELSA - Collaborative research project - Application form
... Complex pharmaceuticals often have the desired medical effect only in one ‘enantiomeric’ form, while the mirror image may have no or even adverse effects. Biocatalysts like enzymes often succeed to transform selectively 1 of the 2 enantiomers of a precursor to a desired product, a process termed ‘Ki ...
... Complex pharmaceuticals often have the desired medical effect only in one ‘enantiomeric’ form, while the mirror image may have no or even adverse effects. Biocatalysts like enzymes often succeed to transform selectively 1 of the 2 enantiomers of a precursor to a desired product, a process termed ‘Ki ...
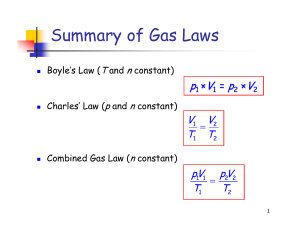
Summary of Gas Laws
... Gas phase in the form of bubbles is formed within the volume of the liquid and the bubbles rise to the surface and burst releasing the vapor into the air This process is called boiling The boiling point of a liquid is increased at higher atmospheric pressures and decreased at lower atmospheric press ...
... Gas phase in the form of bubbles is formed within the volume of the liquid and the bubbles rise to the surface and burst releasing the vapor into the air This process is called boiling The boiling point of a liquid is increased at higher atmospheric pressures and decreased at lower atmospheric press ...
Unit 7: Chemical Equations & Reactions
... • Adjust the coefficients to obtain the same number of atoms of this element on both sides. • Balance polyatomic ions as a unit (if possible). • Re-write H2O as H-OH if hydroxide is present 3. Balance the remaining atoms • End with the least-complex substance ...
... • Adjust the coefficients to obtain the same number of atoms of this element on both sides. • Balance polyatomic ions as a unit (if possible). • Re-write H2O as H-OH if hydroxide is present 3. Balance the remaining atoms • End with the least-complex substance ...
Hydrogen Chemistry of Basalt Aquifers -- Treiman et
... water. This was only inferred from isotopic measurements and several assumptions about isotope equilibrium and exchange. Direct experimental measurements of rock-water interaction with materials from this site are required in order to prove the source of hydrogen in this system. It is also important ...
... water. This was only inferred from isotopic measurements and several assumptions about isotope equilibrium and exchange. Direct experimental measurements of rock-water interaction with materials from this site are required in order to prove the source of hydrogen in this system. It is also important ...
Homework Assignment #4
... For the following exercises, you will need a periodic table. Check the course web site for useful links. 7. (4 pts) The amino acid methionine has the molecular formula of C5H11NO2S. a) Its molecular weight is: ...
... For the following exercises, you will need a periodic table. Check the course web site for useful links. 7. (4 pts) The amino acid methionine has the molecular formula of C5H11NO2S. a) Its molecular weight is: ...
File - IGCSE STUDY BANK
... I was once asked "what is the opposite of a catalyst?" There is no real opposite to a catalyst, other than the uncatalysed reaction! The word catalyst means changing the rate of a reaction with some other material 'added to' or in 'contact with' the reaction mixture. There are the two phrases you ma ...
... I was once asked "what is the opposite of a catalyst?" There is no real opposite to a catalyst, other than the uncatalysed reaction! The word catalyst means changing the rate of a reaction with some other material 'added to' or in 'contact with' the reaction mixture. There are the two phrases you ma ...
Semester II Exam Review Questions
... 13. What is the boiling point temperature for Yummygum when the external pressure is 75 atmospheres? 14. What is the freezing point temperature for Yummygum when the external pressure is 70 atmospheres? 15. If you were to have a container of Yummygum in your kitchen, in what state (phase of matter) ...
... 13. What is the boiling point temperature for Yummygum when the external pressure is 75 atmospheres? 14. What is the freezing point temperature for Yummygum when the external pressure is 70 atmospheres? 15. If you were to have a container of Yummygum in your kitchen, in what state (phase of matter) ...
1 Introduction
... and/or transporting these highly toxic reagents is problematic. To understand this better, let us look at two examples: hydrogen-powered fuel cells and oxidation of propene to propene oxide. Hydrogen-powered fuel cells are a hot topic. In principle, such fuel cells can provide us with energy, whil ...
... and/or transporting these highly toxic reagents is problematic. To understand this better, let us look at two examples: hydrogen-powered fuel cells and oxidation of propene to propene oxide. Hydrogen-powered fuel cells are a hot topic. In principle, such fuel cells can provide us with energy, whil ...
Industrial Chemicals Technology Hand Book
... Growth in demand for chemicals in developing countries is high leading to substantial cross border investment in the chemical sector. The chemical industry comprises the companies that produce industrial chemicals. Chemicals are used to make a wide variety of consumer goods, as well as thousands inp ...
... Growth in demand for chemicals in developing countries is high leading to substantial cross border investment in the chemical sector. The chemical industry comprises the companies that produce industrial chemicals. Chemicals are used to make a wide variety of consumer goods, as well as thousands inp ...
Chapter 1: Matter, Measurement and Problem Solving
... Molecules have a lot of space between them Free to move Compressible: forcing gas into a small container No fixed volume: volume of the container No fixed shape: take the shape of container ...
... Molecules have a lot of space between them Free to move Compressible: forcing gas into a small container No fixed volume: volume of the container No fixed shape: take the shape of container ...
Mid Term Exam Topics 1-5 solution - OCW
... For water, the density of the solid is smaller than for the liquid so the slope is negative. But this is not the case for carbon dioxide which has a positive slope. c) From 218 to 1 atm, water exists as a liquid. At 1 atm, it transforms in solid and remains as a solid until pressure reaches a value ...
... For water, the density of the solid is smaller than for the liquid so the slope is negative. But this is not the case for carbon dioxide which has a positive slope. c) From 218 to 1 atm, water exists as a liquid. At 1 atm, it transforms in solid and remains as a solid until pressure reaches a value ...
Catalytic reforming

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.In addition to a gasoline blending stock, reformate is the main source of aromatic bulk chemicals such as benzene, toluene, xylene and ethylbenzene which have diverse uses, most importantly as raw materials for conversion into plastics. However, the benzene content of reformate makes it carcinogenic, which has led to governmental regulations effectively requiring further processing to reduce its benzene content.This process is quite different from and not to be confused with the catalytic steam reforming process used industrially to produce products such as hydrogen, ammonia, and methanol from natural gas, naphtha or other petroleum-derived feedstocks. Nor is this process to be confused with various other catalytic reforming processes that use methanol or biomass-derived feedstocks to produce hydrogen for fuel cells or other uses.