Basic Stoichometry
... you made a batch of cookies and used way too many eggs, or not enough sugar. YUCK! In chemistry, reactions proceed with very specific recipes. The study of these recipes is stoichiometry. When the reactants are present in the correct amounts, the reaction will produce products. What happens if there ...
... you made a batch of cookies and used way too many eggs, or not enough sugar. YUCK! In chemistry, reactions proceed with very specific recipes. The study of these recipes is stoichiometry. When the reactants are present in the correct amounts, the reaction will produce products. What happens if there ...
Predictive thermodynamics for ionic solids and
... compounds.2 Ionic liquids are combinations of such a broad range of cations and anions so that the in-principle possible number of such liquids is of the order of 1018 (although the realistically possible number is orders of magnitude smaller) – already, some 1000 have been reported in the literatur ...
... compounds.2 Ionic liquids are combinations of such a broad range of cations and anions so that the in-principle possible number of such liquids is of the order of 1018 (although the realistically possible number is orders of magnitude smaller) – already, some 1000 have been reported in the literatur ...
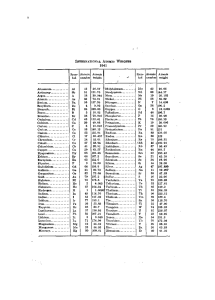
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
Ternary nucleation of inorganic acids, ammonia, and water
... corresponding to the reference pressure 1 atm. The equilibrium constant for sulfuric acid dihydrate formation K 1 K 2 is estimated, as explained in Ref. 16. The formation energy of a dihydrate according to the classical droplet model ⌬G(2,1,0) is calculated from Eq. 共1兲. The H2 O–H2 SO4 dihydrate is ...
... corresponding to the reference pressure 1 atm. The equilibrium constant for sulfuric acid dihydrate formation K 1 K 2 is estimated, as explained in Ref. 16. The formation energy of a dihydrate according to the classical droplet model ⌬G(2,1,0) is calculated from Eq. 共1兲. The H2 O–H2 SO4 dihydrate is ...
Educator`s Reference Guide for Electrochemistry
... Of the three main divisions of analytical chemistry–spectroscopy, chromatography, and electrochemistry–the latter generally receives the least attention in an undergraduate instrumental analysis course, in both the lecture and the laboratory. In many cases, undergraduate students complete their degr ...
... Of the three main divisions of analytical chemistry–spectroscopy, chromatography, and electrochemistry–the latter generally receives the least attention in an undergraduate instrumental analysis course, in both the lecture and the laboratory. In many cases, undergraduate students complete their degr ...
Version A
... 52. When dilute nitric acid was added to a solution of one of the following chemicals, a gas was evolved, This gas turned a drop of limewater, Ca(OH)2, cloudy, due to the formation of a white precipitate. The chemical was ...
... 52. When dilute nitric acid was added to a solution of one of the following chemicals, a gas was evolved, This gas turned a drop of limewater, Ca(OH)2, cloudy, due to the formation of a white precipitate. The chemical was ...
PX312-1718
... 25. Sodium chloride is added slowly to a solution that is 0.010 M in Cu+, Ag+, and Au+. The Ksp values for the chloride salts are 1.9 10–7, 1.6 10–10, and 2.0 10–13, respectively. Which compound will precipitate first? A) AuCl(s) B) All will precipitate at the same time. C) It cannot be determ ...
... 25. Sodium chloride is added slowly to a solution that is 0.010 M in Cu+, Ag+, and Au+. The Ksp values for the chloride salts are 1.9 10–7, 1.6 10–10, and 2.0 10–13, respectively. Which compound will precipitate first? A) AuCl(s) B) All will precipitate at the same time. C) It cannot be determ ...
Self-complementary double-stranded porphyrin
... 3. The self-complementary coordination bonds were signicantly stabilized by the zipper effect, thereby thermodynamically funnelling the self-assembled structures into the most stable form without kinetic entrapment by metastable states. This allowed for the realization of the specic double-strand f ...
... 3. The self-complementary coordination bonds were signicantly stabilized by the zipper effect, thereby thermodynamically funnelling the self-assembled structures into the most stable form without kinetic entrapment by metastable states. This allowed for the realization of the specic double-strand f ...
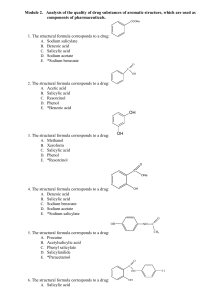
Module 2. Drug substances of aromatic structure
... C. 2-Hydroxybenzenecarboxylic acid D. Oxybenzene E. *2-Hydroxybenzenecarboxylate 56. In the medical practice salicylic acid use as means: A. Purgative B. Anti-inflammatory; analgesic C. Expectorant D. Antimicrobial preservative E. *Keratolytic 57. What indicator must be used, according to Pharmacopo ...
... C. 2-Hydroxybenzenecarboxylic acid D. Oxybenzene E. *2-Hydroxybenzenecarboxylate 56. In the medical practice salicylic acid use as means: A. Purgative B. Anti-inflammatory; analgesic C. Expectorant D. Antimicrobial preservative E. *Keratolytic 57. What indicator must be used, according to Pharmacopo ...
Solving General Chemistry Problems 5e
... position shown below by the small x, between the digits 3 and 5. This gives as the first step ...
... position shown below by the small x, between the digits 3 and 5. This gives as the first step ...
Enthalpy Barriers for Asymmetric SN2 Alkyl
... are in good agreement with those calculated using G3(MP2) theory and are on average lower by 2.5 ( 3.4 kJ mol-1. More importantly, these values are also in good agreement with those experimental values determined by us15,16 being lower on average by 3.7 ( 2.2 kJ mol-1. This lends confidence to the p ...
... are in good agreement with those calculated using G3(MP2) theory and are on average lower by 2.5 ( 3.4 kJ mol-1. More importantly, these values are also in good agreement with those experimental values determined by us15,16 being lower on average by 3.7 ( 2.2 kJ mol-1. This lends confidence to the p ...
Chemical Quantities
... antacid. The company claims that its product neutralizes 10 times as much stomach acid per tablet as its nearest competitor. How would you test the validity of this claim? Or suppose that after graduation you go to work for a chemical company that makes methanol (methyl alcohol), a substance used as ...
... antacid. The company claims that its product neutralizes 10 times as much stomach acid per tablet as its nearest competitor. How would you test the validity of this claim? Or suppose that after graduation you go to work for a chemical company that makes methanol (methyl alcohol), a substance used as ...
A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide
... biosensor had a fast response of less than 10 s to H2O2, with a linear range of 3.5×10-5 to 1.1×10-3 M, and a detection limit of 8.0×10-6 M based on S/N = 3. Keywords: horseradish peroxidase, biosensor, hydrogen peroxide, chitosan ...
... biosensor had a fast response of less than 10 s to H2O2, with a linear range of 3.5×10-5 to 1.1×10-3 M, and a detection limit of 8.0×10-6 M based on S/N = 3. Keywords: horseradish peroxidase, biosensor, hydrogen peroxide, chitosan ...
The Project Gutenberg eBook #50880: Treatise on Thermodynamics.
... B and C are in thermal equilibrium with one another.∗ For, if we bring A, B, and C together so that each touches the other two, then, according to our supposition, there will be equilibrium at the points of contact AB and AC, and, therefore, also at the contact BC. If it were not so, no general ther ...
... B and C are in thermal equilibrium with one another.∗ For, if we bring A, B, and C together so that each touches the other two, then, according to our supposition, there will be equilibrium at the points of contact AB and AC, and, therefore, also at the contact BC. If it were not so, no general ther ...
Stoichiometry - Mr Field's Chemistry Class
... Example 3: How many atoms of hydrogen are there in 2.5 moles of methane (CH4)? •N(H) = 4 x N(CH4) •N(H) = 4 x n(CH4) x L •N(H) = 4 x 2.5 x 6.02x1023 •N(H) = 6.02x1024 ...
... Example 3: How many atoms of hydrogen are there in 2.5 moles of methane (CH4)? •N(H) = 4 x N(CH4) •N(H) = 4 x n(CH4) x L •N(H) = 4 x 2.5 x 6.02x1023 •N(H) = 6.02x1024 ...