... CHAPTER 12, Stoichiometry (continued) 4. If the quantities of reactants are given in units other than moles, what is the first step for determining the amount of product? a. Determine the amount of product from the given amount of limiting reagent. b. Convert each given quantity of reactant to moles ...
Kinetic investigation of low-pH Fe(II) oxidation and development of a
... The removal of H2S from gaseous streams is required for the health of the general public, occupational safety and for operational reasons. The Liquid Redox Sulfur Recovery (LRSR) process is a common and promising process for H2S(g) removal that is based on reactive absorption (i.e. absorption of the ...
... The removal of H2S from gaseous streams is required for the health of the general public, occupational safety and for operational reasons. The Liquid Redox Sulfur Recovery (LRSR) process is a common and promising process for H2S(g) removal that is based on reactive absorption (i.e. absorption of the ...
Limiting Reactants and Percentage Yield
... One reactant limits the product of a reaction. Once one of the reactants is used up, no more product can be formed. The substance that is completely used up first in a reaction is called the limiting reactant. The limiting reactant is the reactant that limits the amount of the other reactant that ca ...
... One reactant limits the product of a reaction. Once one of the reactants is used up, no more product can be formed. The substance that is completely used up first in a reaction is called the limiting reactant. The limiting reactant is the reactant that limits the amount of the other reactant that ca ...
The Control of Moisture of Rocks by Methods of
... ABSTRACT. Laboratory and on-line resonator moisture meters have been developed for application in mining. Metrologic characteristics of developed devices have been studied.. Industrial testing was carried out on a number of rock and chemical materials. The applicability of linear model is shown at t ...
... ABSTRACT. Laboratory and on-line resonator moisture meters have been developed for application in mining. Metrologic characteristics of developed devices have been studied.. Industrial testing was carried out on a number of rock and chemical materials. The applicability of linear model is shown at t ...
HSC Chemistry Syllabus Notes 2007
... 3. Manufactured products, including food, drugs and household chemicals, are analysed to determine or ensure their chemical composition66 4. Human activity has caused changes in the composition and the structure of the atmosphere. Chemists monitor these changes so that further damage can be limited ...
... 3. Manufactured products, including food, drugs and household chemicals, are analysed to determine or ensure their chemical composition66 4. Human activity has caused changes in the composition and the structure of the atmosphere. Chemists monitor these changes so that further damage can be limited ...
IIT-JEE (Advanced) - Brilliant Public School Sitamarhi
... [Useful when only two reactant are there] By calculating amount of any one product obtained taking each reactant one by one irrespective of other reactants. The one giving least product is limiting reagent. Divide given moles of each reactant by their stoichiometric coefficient, the one with least r ...
... [Useful when only two reactant are there] By calculating amount of any one product obtained taking each reactant one by one irrespective of other reactants. The one giving least product is limiting reagent. Divide given moles of each reactant by their stoichiometric coefficient, the one with least r ...
chemistry - Ethiopian Ministry of Education
... Since chemistry is such an enormous area of science, for convenience it has been divided into disciplines. However, the division is never as clear-cut as it might appear to be. All sciences are related and depend on each other – they are interrelated. All the disciplines of science share information ...
... Since chemistry is such an enormous area of science, for convenience it has been divided into disciplines. However, the division is never as clear-cut as it might appear to be. All sciences are related and depend on each other – they are interrelated. All the disciplines of science share information ...
updated chem cp final review key
... g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop changing (3) reactants and products are both pre ...
... g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop changing (3) reactants and products are both pre ...
Overview Density functional calculations of NMR chemical shifts and
... note that they obtain shielding values which are up to 16 ppm smaller than those of Malkin et al. [18±20], with an increasing tendency for increasing numbers of electrons, and they suggest modifying Malkin's empirical correction term. We can conclude that the discussion about current DFT is all but ...
... note that they obtain shielding values which are up to 16 ppm smaller than those of Malkin et al. [18±20], with an increasing tendency for increasing numbers of electrons, and they suggest modifying Malkin's empirical correction term. We can conclude that the discussion about current DFT is all but ...
Vinnitsa National Pirogov Memorial Medical University Biological
... 1.Write the electronic structure of potassium atom and ion. 2. Write the electronic structure of aluminium atom and Al3+ ion. 3.Write the equations of the below given chain. ...
... 1.Write the electronic structure of potassium atom and ion. 2. Write the electronic structure of aluminium atom and Al3+ ion. 3.Write the equations of the below given chain. ...
CHEM 1212 Principles of Chemistry II Course Study Guide
... 1. Use recall. After you read a page, look away and recall the main ideas. Highlight very little, and never highlight anything you haven’t put in your mind first by recalling. Try recalling main ideas when you are walking to class or in a different room from where you originally learned it. An abili ...
... 1. Use recall. After you read a page, look away and recall the main ideas. Highlight very little, and never highlight anything you haven’t put in your mind first by recalling. Try recalling main ideas when you are walking to class or in a different room from where you originally learned it. An abili ...
Chemistry Bridging Work
... a) How many moles of water are needed to react with 0.03 moles of carbon dioxide? b) How many moles of glucose can you make from 0.03 moles of carbon dioxide? c) How many moles of oxygen can you make from 0.03 moles of carbon dioxide? ...
... a) How many moles of water are needed to react with 0.03 moles of carbon dioxide? b) How many moles of glucose can you make from 0.03 moles of carbon dioxide? c) How many moles of oxygen can you make from 0.03 moles of carbon dioxide? ...
Chapter 4
... and [SO4 2-] = 0.010 M. Example 4.1 illustrates an additional idea about ion concentrations in solution: There is only one concentration for any given ion in a solution, even if the ion has more than one source. Additionally, the total ion concentration in a solution is the sum of the molarities of ...
... and [SO4 2-] = 0.010 M. Example 4.1 illustrates an additional idea about ion concentrations in solution: There is only one concentration for any given ion in a solution, even if the ion has more than one source. Additionally, the total ion concentration in a solution is the sum of the molarities of ...
Precipitation of salts in freezing seawater and ozone depletion
... S. Morin et al.: Precipitation of salts in freezing seawater and ODEs HNO3 and H2 SO4 ) would easily lower the pH enough for Reaction (R1) to proceed at an accelerated rate. Piot and von Glasow (2008) have included this process in their modeling study on the conditions necessary to trigger and sust ...
... S. Morin et al.: Precipitation of salts in freezing seawater and ODEs HNO3 and H2 SO4 ) would easily lower the pH enough for Reaction (R1) to proceed at an accelerated rate. Piot and von Glasow (2008) have included this process in their modeling study on the conditions necessary to trigger and sust ...
Acid-Base
... terms of base strength, OH– > NH3 in 1st reaction; C2H5O– > OH– in 2nd rxn. 1980 A Methylamine CH3NH2, is a weak base that ionizes in solution as shown by the following equation. CH3NH2 + H2O ↔ CH3NH3+ + OH– (a) At 25ºC the percentage ionization in a 0.160 molar solution of CH3NH2 is 4.7%. Calculate ...
... terms of base strength, OH– > NH3 in 1st reaction; C2H5O– > OH– in 2nd rxn. 1980 A Methylamine CH3NH2, is a weak base that ionizes in solution as shown by the following equation. CH3NH2 + H2O ↔ CH3NH3+ + OH– (a) At 25ºC the percentage ionization in a 0.160 molar solution of CH3NH2 is 4.7%. Calculate ...
Chemistry - A Quantitative Science
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
Mead Chemistry Lap 11: Stoichiometry Chapter 12 12.1 Balanced
... • You can use amount of one substance to find the amounts of the other substances • Quantity usually in moles or grams • Stoichiometry: calculations of quantities involved in a chemical reaction ▫ Use balanced equation like a recipe B. Interpreting Chemical Equations • Can use balanced equation to f ...
... • You can use amount of one substance to find the amounts of the other substances • Quantity usually in moles or grams • Stoichiometry: calculations of quantities involved in a chemical reaction ▫ Use balanced equation like a recipe B. Interpreting Chemical Equations • Can use balanced equation to f ...
The Advanced Placement Examination in Chemistry Acid–Base
... terms of base strength, OH– > NH3 in 1st reaction; C2H5O– > OH– in 2nd rxn. 1980 A Methylamine CH3NH2, is a weak base that ionizes in solution as shown by the following equation. CH3NH2 + H2O ↔ CH3NH3+ + OH– (a) At 25ºC the percentage ionization in a 0.160 molar solution of CH3NH2 is 4.7%. Calculate ...
... terms of base strength, OH– > NH3 in 1st reaction; C2H5O– > OH– in 2nd rxn. 1980 A Methylamine CH3NH2, is a weak base that ionizes in solution as shown by the following equation. CH3NH2 + H2O ↔ CH3NH3+ + OH– (a) At 25ºC the percentage ionization in a 0.160 molar solution of CH3NH2 is 4.7%. Calculate ...
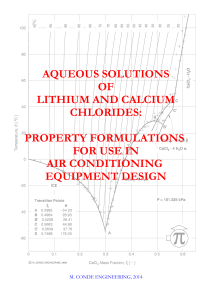
Aqueous solutions of lithium and calcium chlorides
... Formulations derived from basic principles have not done better than the empirical ones and are confined to very dilute solutions. The formulations we report in the following reproduce quite accurately the known experimental data and default to congruent values at the boundaries. We formulate it in ...
... Formulations derived from basic principles have not done better than the empirical ones and are confined to very dilute solutions. The formulations we report in the following reproduce quite accurately the known experimental data and default to congruent values at the boundaries. We formulate it in ...