ExamView - 1999 AP Chemistry Exam.tst
... When the equation above is balanced and all coefficients are reduced to their lowest whole-number terms, the coefficient for O2(g) is? A) 6 B) 7 C) 12 D) 14 E) 28 27. Appropriate uses of a visible-light spectrophotometer include which of the following? I. Determining the concentration of a solution ...
... When the equation above is balanced and all coefficients are reduced to their lowest whole-number terms, the coefficient for O2(g) is? A) 6 B) 7 C) 12 D) 14 E) 28 27. Appropriate uses of a visible-light spectrophotometer include which of the following? I. Determining the concentration of a solution ...
Hydrogen dissociation under equilibrium and non
... chemical reactions. The phase diagram of hydrogen shows that the molecular fluid (H2 ) dominates up to approximately 5000 K. Above that, hydrogen spontaneously dissociates to an atomic fluid. Thus, at “normal” temperatures and pressures hydrogen is present as a molecular fluid, while high temperatur ...
... chemical reactions. The phase diagram of hydrogen shows that the molecular fluid (H2 ) dominates up to approximately 5000 K. Above that, hydrogen spontaneously dissociates to an atomic fluid. Thus, at “normal” temperatures and pressures hydrogen is present as a molecular fluid, while high temperatur ...
enthalpy changes
... is affected to the number of moles of reactant or product. If given the mass of reactant or product, the enthalpy change (∆H in kJ) can be determined from the molar enthalpy value for the ...
... is affected to the number of moles of reactant or product. If given the mass of reactant or product, the enthalpy change (∆H in kJ) can be determined from the molar enthalpy value for the ...
Molecular Level Models for CO2 Sorption in
... the grand canonical Monte Carlo (GCMC) simulations and the nonlocal density functional theory (NLDFT). Three molecular models of CO2 have been used. Long-run GCMC simulations were performed with the three-center model of Harris and Yung (J. Phys. Chem. 1995, 99, 12021). For NLDFT calculations, we de ...
... the grand canonical Monte Carlo (GCMC) simulations and the nonlocal density functional theory (NLDFT). Three molecular models of CO2 have been used. Long-run GCMC simulations were performed with the three-center model of Harris and Yung (J. Phys. Chem. 1995, 99, 12021). For NLDFT calculations, we de ...
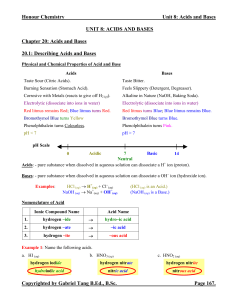
1970 - 2005 Acid/Base FRQs
... A 5.00 gram sample of a dry mixture of potassium hysame and therefore only a very small change in droxide, potassium carbonate, and potassium chloride pH. is reacted with 0.100 liter of 2.00 molar HCl solution NH3 + H+ <=> NH4+ (a) A 249 milliliter sample of dry CO2 gas, measured at 22ºC and 740 tor ...
... A 5.00 gram sample of a dry mixture of potassium hysame and therefore only a very small change in droxide, potassium carbonate, and potassium chloride pH. is reacted with 0.100 liter of 2.00 molar HCl solution NH3 + H+ <=> NH4+ (a) A 249 milliliter sample of dry CO2 gas, measured at 22ºC and 740 tor ...
Effects of Electrolyte Concentration and pH on
... therefore, explained with their effect on the surface forces, e.g., by modifying the electrical potential of drop surface12,22 and the van der Waals interactions,36,37 and by creating a steric barrier to drop-drop coalescence.22,31,35 In other words, it is assumed in these studies that the main role ...
... therefore, explained with their effect on the surface forces, e.g., by modifying the electrical potential of drop surface12,22 and the van der Waals interactions,36,37 and by creating a steric barrier to drop-drop coalescence.22,31,35 In other words, it is assumed in these studies that the main role ...
File - Roden`s AP Chemistry
... to shift to the left (LeChatelier’s principle) where the NH4Cl = 53.5) concentrations of the ions in solution decrease and less (b) If this NH4Cl solution is assumed to be ideal and is can dissolve. completely dissociated into ions, calculate the The diverse (“uncommon”) ion effect – “the salt efpre ...
... to shift to the left (LeChatelier’s principle) where the NH4Cl = 53.5) concentrations of the ions in solution decrease and less (b) If this NH4Cl solution is assumed to be ideal and is can dissolve. completely dissociated into ions, calculate the The diverse (“uncommon”) ion effect – “the salt efpre ...
GEOCHEMICAL AND BIOGEOCHEMICAL
... calculation. Ming-Kuo Lee and I added Pitzer’s activity model and a method for tracing isotope fractionation. Twice I replaced GTPLOT with new, more powerful programs. I wrote ACT 2 and TACT to produce activity–activity and temperature– activity diagrams, and RXN to balance reactions and compute equ ...
... calculation. Ming-Kuo Lee and I added Pitzer’s activity model and a method for tracing isotope fractionation. Twice I replaced GTPLOT with new, more powerful programs. I wrote ACT 2 and TACT to produce activity–activity and temperature– activity diagrams, and RXN to balance reactions and compute equ ...
Chapter 14
... Third law of thermodynamics: The absolute entropy (S) of a perfect crystal of any pure substance at absolute zero is 0.0 J/mol.K. Because there are standard ways of find the change in entropy for a pure substance as we change the temperature of the substance at constant pressure, the third law of t ...
... Third law of thermodynamics: The absolute entropy (S) of a perfect crystal of any pure substance at absolute zero is 0.0 J/mol.K. Because there are standard ways of find the change in entropy for a pure substance as we change the temperature of the substance at constant pressure, the third law of t ...
Thermodynamics: Entropy and Free Energy
... standard molar entropy, J/mol K. Table 19.1.1 shows standard molar entropy values for water at various temperatures. Interactive Figure 19.1.11 shows a generalized plot of the entropy of a substance at each temperature from 0 K to above its boiling point. Note in Table 19.1.1 and Interactive Figur ...
... standard molar entropy, J/mol K. Table 19.1.1 shows standard molar entropy values for water at various temperatures. Interactive Figure 19.1.11 shows a generalized plot of the entropy of a substance at each temperature from 0 K to above its boiling point. Note in Table 19.1.1 and Interactive Figur ...
Cold interactions between an Yb ion and a Li atom
... Before presenting our potential-energy curves, and permanent and transition dipole moments, we first compare the computed atomic results to the best available experimental data. Our predicted position of the nonrelativistic 2 P state of the Li atom is 14 910 cm−1 , to be compared with the experiment ...
... Before presenting our potential-energy curves, and permanent and transition dipole moments, we first compare the computed atomic results to the best available experimental data. Our predicted position of the nonrelativistic 2 P state of the Li atom is 14 910 cm−1 , to be compared with the experiment ...
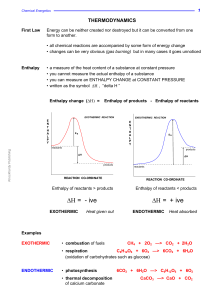
Η - Knockhardy
... The enthalpy change when ONE MOLE of a compound is formed from its elements. Standard Enthalpy Change of Formation ( ∆Η°f) Definition ...
... The enthalpy change when ONE MOLE of a compound is formed from its elements. Standard Enthalpy Change of Formation ( ∆Η°f) Definition ...
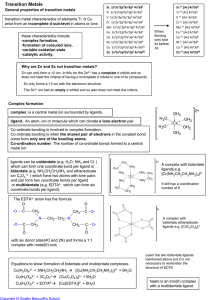
Transition Metals
... Reducing Chromium Cr3+ (green) and Cr2+ (blue) are formed by reduction of Cr2O72(orange) by zinc in acid solution, but Fe2+ will only reduce it to Cr3+ The Fe2+ and Cr2O7 2- in acid solution reaction can be used as a quantitative redox titration. This does not need an indicator Manganate redox titr ...
... Reducing Chromium Cr3+ (green) and Cr2+ (blue) are formed by reduction of Cr2O72(orange) by zinc in acid solution, but Fe2+ will only reduce it to Cr3+ The Fe2+ and Cr2O7 2- in acid solution reaction can be used as a quantitative redox titration. This does not need an indicator Manganate redox titr ...
Stoichiometry - HCC Learning Web
... Since the moles of FeCl3 based on moles of Cl2 is the smaller answer, Cl2 is the limiting reactant. Iron metal is therefore in excess amount, so there will be some Fe left over unreacted. Note that we might have reasonably assumed that iron metal was the limiting reactant since it was present in les ...
... Since the moles of FeCl3 based on moles of Cl2 is the smaller answer, Cl2 is the limiting reactant. Iron metal is therefore in excess amount, so there will be some Fe left over unreacted. Note that we might have reasonably assumed that iron metal was the limiting reactant since it was present in les ...
The Major Classes of Chemical Reactions
... Chemists use three types of equations to represent aqueous ionic reactions: molecular, total ionic, and net ionic equations. As you’ll see in the two types of ionic equations, by balancing the atoms, we also balance the charges. Let’s examine a reaction to see what each of these equations shows. Whe ...
... Chemists use three types of equations to represent aqueous ionic reactions: molecular, total ionic, and net ionic equations. As you’ll see in the two types of ionic equations, by balancing the atoms, we also balance the charges. Let’s examine a reaction to see what each of these equations shows. Whe ...