Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... Law of Conservation of Mass “We may lay it down as an incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical ...
... Law of Conservation of Mass “We may lay it down as an incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical ...
Predicting point defect equilibria across oxide hetero-interfaces
... of semiconductor engineering. The drift-diffusion model is welldeveloped for semiconductor heterojunctions,12–14,32,33 where only electrons and holes are considered as the mobile species. In that case, there is no need to consider segregation energies since free holes and electrons are assumed to be ...
... of semiconductor engineering. The drift-diffusion model is welldeveloped for semiconductor heterojunctions,12–14,32,33 where only electrons and holes are considered as the mobile species. In that case, there is no need to consider segregation energies since free holes and electrons are assumed to be ...
Homogeneous Catalysis
... Chemical Kinetics is the study of reaction rates; that is, how fast a given reaction does proceeds. It is a measure of the change of the concentration of reactants (or products) as a function of time. Reaction rates provide information regarding how fast a chemical process occurs as well as the mech ...
... Chemical Kinetics is the study of reaction rates; that is, how fast a given reaction does proceeds. It is a measure of the change of the concentration of reactants (or products) as a function of time. Reaction rates provide information regarding how fast a chemical process occurs as well as the mech ...
chemistry - Textbooks Online
... and Marie Lavoisier and by John Dalton on the chemistry of air and the atomic nature of matter paved the way for modern chemistry. During the nineteenth century chemists worked steadily towards an understanding of the relationships between the different chemical elements and the way they react toget ...
... and Marie Lavoisier and by John Dalton on the chemistry of air and the atomic nature of matter paved the way for modern chemistry. During the nineteenth century chemists worked steadily towards an understanding of the relationships between the different chemical elements and the way they react toget ...
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
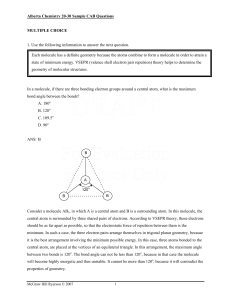
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
A comparison of the effects of fluoride and chloride
... This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of ...
... This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of ...
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... • Recognizing the existence of hydrogen bonds ( between molecules) (1) • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
... • Recognizing the existence of hydrogen bonds ( between molecules) (1) • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
Chapter 5 Geochemical Weathering
... Weathering of landscapes involves an array of mechanical and geochemical agents that conspire to alter primary geological formations to sediments and solutes. Geochemical weathering is driven by water. In soils, water is the limiting factor for the activity of aerobic bacteria that degrade organics ...
... Weathering of landscapes involves an array of mechanical and geochemical agents that conspire to alter primary geological formations to sediments and solutes. Geochemical weathering is driven by water. In soils, water is the limiting factor for the activity of aerobic bacteria that degrade organics ...
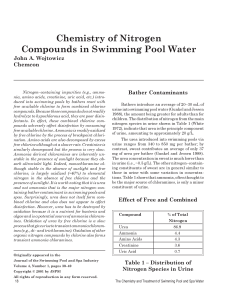
w_4-3 Chemistry of Nitrogen Compounds
... free available chlorine. Ammonia is readily oxidized by free chlorine by the process of breakpoint chlorination. Amino acids are also decomposed by excess free chlorine although at a slower rate. Creatinine is similarly decomposed but the process is very slow. Ammonia derived chloramines are inheren ...
... free available chlorine. Ammonia is readily oxidized by free chlorine by the process of breakpoint chlorination. Amino acids are also decomposed by excess free chlorine although at a slower rate. Creatinine is similarly decomposed but the process is very slow. Ammonia derived chloramines are inheren ...
Post Lab Questions
... Policy for making up missed work and turning in late assignments: If you are going to miss class please inform me with an email. Your opportunity to make up work is dependent on written approval. In general most missed work is due on the next Friday. For any planned absence work that is due during t ...
... Policy for making up missed work and turning in late assignments: If you are going to miss class please inform me with an email. Your opportunity to make up work is dependent on written approval. In general most missed work is due on the next Friday. For any planned absence work that is due during t ...
- Wiley Online Library
... rate calculations. Furthermore, the standard CNT for ion-induced binary system [Yue and Chan, 1979] has been criticized for the fact that it cannot differentiate between the enhancement of formation rates due to negative and positive ions [Lovejoy et al., 2004; Sorokin et al., 2006]. These shortcomin ...
... rate calculations. Furthermore, the standard CNT for ion-induced binary system [Yue and Chan, 1979] has been criticized for the fact that it cannot differentiate between the enhancement of formation rates due to negative and positive ions [Lovejoy et al., 2004; Sorokin et al., 2006]. These shortcomin ...
On inferring isoprene emission surface flux from atmospheric
... initial and boundary layer values prescribed for the reference case. These values are similar to those discussed by Betts and Jacob (2002) gathered during the Large-Scale BiosphereAtmosphere (LBA) experiment in Amazonia. Figure 1 shows the prescribed values of the surface fluxes and the calculated e ...
... initial and boundary layer values prescribed for the reference case. These values are similar to those discussed by Betts and Jacob (2002) gathered during the Large-Scale BiosphereAtmosphere (LBA) experiment in Amazonia. Figure 1 shows the prescribed values of the surface fluxes and the calculated e ...
Module 3: Defects, Diffusion and Conduction in Ceramics
... concentration i.e. [V Na '] = [Cd Na •] . The diffusivity is given by ...
... concentration i.e. [V Na '] = [Cd Na •] . The diffusivity is given by ...
Computational Study of Polarizabilities and Second
... more serious for 〈γ〉vacuum than for 〈R〉vacuum. From this point of view, modeling NLO properties in solution is not only important for assessing solvent effects upon NLO properties of interest, but it also provides the only reliable way to model and predict NLO properties for a series of anionic inor ...
... more serious for 〈γ〉vacuum than for 〈R〉vacuum. From this point of view, modeling NLO properties in solution is not only important for assessing solvent effects upon NLO properties of interest, but it also provides the only reliable way to model and predict NLO properties for a series of anionic inor ...
Unit 3 4 Balancing Chemical Reaction Equations by Inspection
... Before going on … I must introduce to you a little something called the polyatomic ions. (Review: recall that the terms ion ≠ ionic) B) Polyatomic ion: A chemically unstable group of bonded atoms, which end up with an unequal number of protons and electrons. Since most PAI are made of bonded nonmet ...
... Before going on … I must introduce to you a little something called the polyatomic ions. (Review: recall that the terms ion ≠ ionic) B) Polyatomic ion: A chemically unstable group of bonded atoms, which end up with an unequal number of protons and electrons. Since most PAI are made of bonded nonmet ...
2003 AP Chemistry Form B Scoring Guidelines - AP Central
... (d) On the graph above, make a sketch that shows how the concentration of H2(g) changes as a function of time. From the graph, [H2]eq is 0.10 M The curve should have the following characteristics: - start at 0 M; - increase to 0.1 M; - reach equilibrium at the same time [HI] reaches equilibrium ...
... (d) On the graph above, make a sketch that shows how the concentration of H2(g) changes as a function of time. From the graph, [H2]eq is 0.10 M The curve should have the following characteristics: - start at 0 M; - increase to 0.1 M; - reach equilibrium at the same time [HI] reaches equilibrium ...