Operating Principles
... There are three types of electron transitions, as shown in figure 2. The first type of transition, shown in figure 2 (a), is known as resonant absorption. An electron transits from the stable low energy level, E1, to the higher energy level, E2, by absorbing light. Figure 2 (b) shows spontaneous emi ...
... There are three types of electron transitions, as shown in figure 2. The first type of transition, shown in figure 2 (a), is known as resonant absorption. An electron transits from the stable low energy level, E1, to the higher energy level, E2, by absorbing light. Figure 2 (b) shows spontaneous emi ...
Lecture 13: Heisenberg and Uncertainty
... tweezer. Either way, the researchers can use that behavior to scoot particles where they want them to go. ...
... tweezer. Either way, the researchers can use that behavior to scoot particles where they want them to go. ...
ic199p5a
... from this list to have these two other structures and briefly explain the reason for your choice (assume that the anion is bigger than the cation in each case). Ans.: BeSe is most likely to have the zinc blende structure and CsBr the cesium chloride structure, because the radius ratio determines whi ...
... from this list to have these two other structures and briefly explain the reason for your choice (assume that the anion is bigger than the cation in each case). Ans.: BeSe is most likely to have the zinc blende structure and CsBr the cesium chloride structure, because the radius ratio determines whi ...
Physics 121 Hour Exam #5 Solution This exam consists of a five
... 2. [12 points] Can a photon (particle of light) decay into a single particle which has mass (m > 0)? Briefly justify your answer. No. This would violate the conservation of momentum and energy. Here’s one way to look at it. Let’s say that the photon is in the Home Frame, moving to the right, and it ...
... 2. [12 points] Can a photon (particle of light) decay into a single particle which has mass (m > 0)? Briefly justify your answer. No. This would violate the conservation of momentum and energy. Here’s one way to look at it. Let’s say that the photon is in the Home Frame, moving to the right, and it ...
Luminescence spectroscopy
... Electrons crossing the singlet state to the triplet state with a flipped spin can also follow one of three choices including returning to the singlet state (including a flip in spin), relax to ground state by internal or/and external conversion, or lose their energy as a photon (phosphorescence, Ph) ...
... Electrons crossing the singlet state to the triplet state with a flipped spin can also follow one of three choices including returning to the singlet state (including a flip in spin), relax to ground state by internal or/and external conversion, or lose their energy as a photon (phosphorescence, Ph) ...
Photons and Matter Waves
... a metal surface, a stream of electrons emerges from the metal. The interference filter is then replaced with one transmitting at 300 nm and the lamp adjusted so that the intensity of the light striking the surface is the same as it was for the 400-nm light. With the 300-nm light, 1) more electrons a ...
... a metal surface, a stream of electrons emerges from the metal. The interference filter is then replaced with one transmitting at 300 nm and the lamp adjusted so that the intensity of the light striking the surface is the same as it was for the 400-nm light. With the 300-nm light, 1) more electrons a ...
Period 3 Activity Solutions: Electromagnetic Waves – Radiant Energy II
... b) Which photon has a larger frequency, the absorbed or either of the emitted photon? How do you know? The absorbed photon (photon #1) has a higher frequency. Photons with greater energy have a higher frequency. c) Which photon has a longer wavelength – the absorbed or the emitted photon? How do you ...
... b) Which photon has a larger frequency, the absorbed or either of the emitted photon? How do you know? The absorbed photon (photon #1) has a higher frequency. Photons with greater energy have a higher frequency. c) Which photon has a longer wavelength – the absorbed or the emitted photon? How do you ...
document
... promotion of one or more electrons to higher energy levels. That is, electromagnetic radiation is absorbed by the atom, which is converted from its ground to one of many possible excited states. • Since the energy of electrons in orbital is fixed, it should be clear that when an electron is promoted ...
... promotion of one or more electrons to higher energy levels. That is, electromagnetic radiation is absorbed by the atom, which is converted from its ground to one of many possible excited states. • Since the energy of electrons in orbital is fixed, it should be clear that when an electron is promoted ...
Chapter 4 Optical Sources
... population of atoms in the upper energy level is greater than that of the lower energy level (i.e. N2 > N1) This condition is known as population inversion and achieved using an external energy source and also known as ‘pumping’. ...
... population of atoms in the upper energy level is greater than that of the lower energy level (i.e. N2 > N1) This condition is known as population inversion and achieved using an external energy source and also known as ‘pumping’. ...
Early observations
... Albert Einstein's mathematical description in 1905 of how it was caused by absorption of what were later called photons, or quanta of light, in the interaction of light with the electrons in the substance, was contained in the paper named "On a Heuristic Viewpoint Concerning the Production and Trans ...
... Albert Einstein's mathematical description in 1905 of how it was caused by absorption of what were later called photons, or quanta of light, in the interaction of light with the electrons in the substance, was contained in the paper named "On a Heuristic Viewpoint Concerning the Production and Trans ...
An Investigation on the Transition Metal Doping and Energy Band
... In recent years, perovskite-type alkali tantalates, ATaO3 (A = Li, Na, and K) show reasonable activities for water splitting under UV irradiation. However, the alkali tantalates are usually less active under visible-light irradiation [3-4]. The transition metal ions, was found to be attractive dopan ...
... In recent years, perovskite-type alkali tantalates, ATaO3 (A = Li, Na, and K) show reasonable activities for water splitting under UV irradiation. However, the alkali tantalates are usually less active under visible-light irradiation [3-4]. The transition metal ions, was found to be attractive dopan ...
9.4.2 Photoelectric Effect
... Black body: an object that can absorb and/or emit energy perfectly, e.g. tungsten o When a black body is heated in a vacuum, it starts emitting radiation perfectly o Emits all types of radiation (light, IR, UV, etc.) Intensity of this radiation varies with the wavelength, can be plotted o For a give ...
... Black body: an object that can absorb and/or emit energy perfectly, e.g. tungsten o When a black body is heated in a vacuum, it starts emitting radiation perfectly o Emits all types of radiation (light, IR, UV, etc.) Intensity of this radiation varies with the wavelength, can be plotted o For a give ...
Period 3 Solutions: Electromagnetic Waves – Radiant Energy II
... a) Connect a solar cell to the white amplifier/loudspeaker. What happens when an LED flashlight connected to a radio shines on the solar cell? What type of radiant energy transfers information? A modulated (changing) current from the radio transfers information by modulating the amplitude of the bea ...
... a) Connect a solar cell to the white amplifier/loudspeaker. What happens when an LED flashlight connected to a radio shines on the solar cell? What type of radiant energy transfers information? A modulated (changing) current from the radio transfers information by modulating the amplitude of the bea ...
Material Chemistry
... • Also, a significantly broad range of emission covered by many sizes of nanocrystals of a given material can be excited at the same wavelength. The typical line widths are20-30nm, thus relatively narrow, which helps if one wants to use the quantum dots more effectively for multispectral imaging. ...
... • Also, a significantly broad range of emission covered by many sizes of nanocrystals of a given material can be excited at the same wavelength. The typical line widths are20-30nm, thus relatively narrow, which helps if one wants to use the quantum dots more effectively for multispectral imaging. ...
Introducing Photochemistry
... Another revolutionary application of electronically excited molecular systems is in laser technology. Lasers are intense sources of monochromatic and coherent radiation. From their early development in 1960, they have found wide fields of application. They have provided powerful tools for the study ...
... Another revolutionary application of electronically excited molecular systems is in laser technology. Lasers are intense sources of monochromatic and coherent radiation. From their early development in 1960, they have found wide fields of application. They have provided powerful tools for the study ...
Teaching program
... VCE Unit 4 Physics Possible teaching program Due to various reasons the time available to teach unit 4 is limited to 11-12 weeks. Usually teachers struggle to cover the prescribed content and to allow time for revision and exam preparation. The available time is often not enough to provide a practic ...
... VCE Unit 4 Physics Possible teaching program Due to various reasons the time available to teach unit 4 is limited to 11-12 weeks. Usually teachers struggle to cover the prescribed content and to allow time for revision and exam preparation. The available time is often not enough to provide a practic ...
Title: Noncollinear phase matching in nonlinear optics Author
... Abstract: Luminescence in semiconductors can have very fast decay (in terms of picoseconds). Measuring the time evolution of the process requires resolution in terms of hundreds of femtoseconds ( ~ 10~ u s). This high resolution can be reached using optical upconversion method. In the theoretical se ...
... Abstract: Luminescence in semiconductors can have very fast decay (in terms of picoseconds). Measuring the time evolution of the process requires resolution in terms of hundreds of femtoseconds ( ~ 10~ u s). This high resolution can be reached using optical upconversion method. In the theoretical se ...
PE EFFECT - cranson
... A new theory of light: • Electromagnetic waves carry discrete energy packets • The energy per packet depends on wavelength, explaining Lenard’s threshold frequency. • More intense light corresponds to more photons, not higher energy photons. This was published in his famous 1905 paper: “On a Heurist ...
... A new theory of light: • Electromagnetic waves carry discrete energy packets • The energy per packet depends on wavelength, explaining Lenard’s threshold frequency. • More intense light corresponds to more photons, not higher energy photons. This was published in his famous 1905 paper: “On a Heurist ...
105 photoelectric_calc
... 5. A sample of magnesium is used as an electrode in a photocell. It is illuminated with u-v light of wavelength 225 nm, and a current flows in the photocell. The current can be reduced to zero by making the other electrode negative using a potential difference of 1.4 V. Calculate the work function o ...
... 5. A sample of magnesium is used as an electrode in a photocell. It is illuminated with u-v light of wavelength 225 nm, and a current flows in the photocell. The current can be reduced to zero by making the other electrode negative using a potential difference of 1.4 V. Calculate the work function o ...
Particle-Wave Duality
... While we use similar notation for particles and for true waves, various quantities are defined differently. Do not make the mistake of using optical definitions for particles. ...
... While we use similar notation for particles and for true waves, various quantities are defined differently. Do not make the mistake of using optical definitions for particles. ...
Notes on Quantum Theory
... (Note that each photon releases a tiny “pulse” of light, but with so many emissions each second, the resulting light would appear “continuous”.) The Photoelectric Effect In the above example, matter (consisting of large numbers of atoms) gives off light as a result of absorbing energy. It turns out ...
... (Note that each photon releases a tiny “pulse” of light, but with so many emissions each second, the resulting light would appear “continuous”.) The Photoelectric Effect In the above example, matter (consisting of large numbers of atoms) gives off light as a result of absorbing energy. It turns out ...
Module 1 - Identifying Metals Using Atomic Emission
... Students will learn how applying a flame to a metal solution can produce a distinct color of light. Chemists use this technique to determine the quantity of various metals contained in a sample. Introduction: The electrons in an atom occupy different energy levels. At the lowest possible energy ...
... Students will learn how applying a flame to a metal solution can produce a distinct color of light. Chemists use this technique to determine the quantity of various metals contained in a sample. Introduction: The electrons in an atom occupy different energy levels. At the lowest possible energy ...
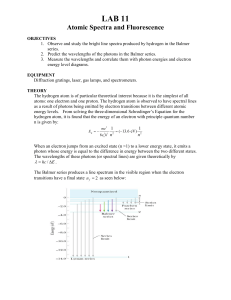
Lab 11: Atomic Spectra
... lower frequencies. For example, an atom can absorb a UV photon and jump to a higher energy state. Rather then jumping directly back to the initial energy state (and emitting the same energy UV photon), the atom can make several smaller jumps and emit lower energy photons (such as visible light). Thi ...
... lower frequencies. For example, an atom can absorb a UV photon and jump to a higher energy state. Rather then jumping directly back to the initial energy state (and emitting the same energy UV photon), the atom can make several smaller jumps and emit lower energy photons (such as visible light). Thi ...