Practice Problem Set #6
... Which has the higher frequency? Which has the highest energy per photon? Calculate the frequency of amber light. 2. Place the following types of radiation in order of increasing energy per photon: a. yellow light from a sodium lamp b. x-rays from an instrument in a dentist’s office c. microwaves i ...
... Which has the higher frequency? Which has the highest energy per photon? Calculate the frequency of amber light. 2. Place the following types of radiation in order of increasing energy per photon: a. yellow light from a sodium lamp b. x-rays from an instrument in a dentist’s office c. microwaves i ...
Quantum Theory - akugakbutuheksis
... An alternate view is that light is acting like a particle The light particle must have sufficient energy to “free” the electron from the atom. Increasing the Amplitude is simply increasing the number of light particles, but its NOT increasing the energy of each one! Increasing the Amplitude ...
... An alternate view is that light is acting like a particle The light particle must have sufficient energy to “free” the electron from the atom. Increasing the Amplitude is simply increasing the number of light particles, but its NOT increasing the energy of each one! Increasing the Amplitude ...
PPT - Tensors for Tots
... exchange of energy between the oscillators and the electromagnetic radiation field occurs in tiny energy packets rather than continuously as in the classical view. Planck's Radiation Law describes the distribution of intensities of electromagnetic energy and power and also the density distribution o ...
... exchange of energy between the oscillators and the electromagnetic radiation field occurs in tiny energy packets rather than continuously as in the classical view. Planck's Radiation Law describes the distribution of intensities of electromagnetic energy and power and also the density distribution o ...
example8
... electromagnetic radiation is shone onto the metal. For this to happen the frequency of the electromagnetic radiation must be above a certain minimum frequency known as the threshold frequency. If the frequency is below this threshold, then no matter how bright the radiation is, the electrons are not ...
... electromagnetic radiation is shone onto the metal. For this to happen the frequency of the electromagnetic radiation must be above a certain minimum frequency known as the threshold frequency. If the frequency is below this threshold, then no matter how bright the radiation is, the electrons are not ...
Chap. 13 -- Atomic P..
... In the diagram above we have represented some allowed energy levels of the electron in a hydrogen atom. Ordinarily, the electron is in the “ground state” (n = 1) and has energy of –13.6 eV. (1 eV = 1.6 10–19 J.) This means that you would have to add 13.6 eV of energy to the electron in order to br ...
... In the diagram above we have represented some allowed energy levels of the electron in a hydrogen atom. Ordinarily, the electron is in the “ground state” (n = 1) and has energy of –13.6 eV. (1 eV = 1.6 10–19 J.) This means that you would have to add 13.6 eV of energy to the electron in order to br ...
Atomic Levels
... H ionization energy ! For galactic sources the Lyman break is far-ultra violet ! which is blocked by earth atmosphere.! For extragalactic sources λobs = (1+z) λemit and ! the limit is shifted in optical and infrared ! ...
... H ionization energy ! For galactic sources the Lyman break is far-ultra violet ! which is blocked by earth atmosphere.! For extragalactic sources λobs = (1+z) λemit and ! the limit is shifted in optical and infrared ! ...
Modern Physics
... If light is like a particle does it have momentum? In Compton scattering x-rays impart momentum to matter, scattering electrons like billiard balls Thus photons also have momentum. The momentum of a photon is given by E hf h ...
... If light is like a particle does it have momentum? In Compton scattering x-rays impart momentum to matter, scattering electrons like billiard balls Thus photons also have momentum. The momentum of a photon is given by E hf h ...
Lava Lamp – Tracing the Energy
... Lava Lamp – Tracing the Energy I know that energy comes in through the electric cord and goes into the light bulb. The energy then goes from the light bulb into the bottle by radiation. The energy is transferred to the wax that rests on the metal ring by conduction. This is because they are in direc ...
... Lava Lamp – Tracing the Energy I know that energy comes in through the electric cord and goes into the light bulb. The energy then goes from the light bulb into the bottle by radiation. The energy is transferred to the wax that rests on the metal ring by conduction. This is because they are in direc ...
Preview of Period 3: Electromagnetic Waves – Radiant Energy II
... photon, the electron is raised to a higher energy level. The electron can emit one or more photons by dropping back to a lower energy level. An electron fluoresces if it absorbs an ultraviolet photon and emits a visible light photon. When a glowing gas is viewed through a diffraction grating, bright ...
... photon, the electron is raised to a higher energy level. The electron can emit one or more photons by dropping back to a lower energy level. An electron fluoresces if it absorbs an ultraviolet photon and emits a visible light photon. When a glowing gas is viewed through a diffraction grating, bright ...
Extended Abstract Template
... Solid phase extraction (SPE) is a widely used extraction method in sample preparation and pre-concentration because of high concentration factor. It is normally performed using either cartridge or disc format. However, commercially available SPE sorbent is relatively expensive and SPE process is les ...
... Solid phase extraction (SPE) is a widely used extraction method in sample preparation and pre-concentration because of high concentration factor. It is normally performed using either cartridge or disc format. However, commercially available SPE sorbent is relatively expensive and SPE process is les ...
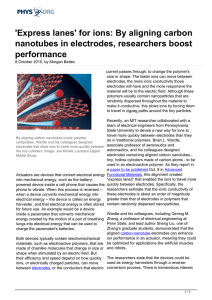
`Express lanes` for ions: By aligning carbon nanotubes in
... aligned carbon-nanotube electrodes can enhance ion performance in an actuator, meaning they could Both devices typically contain electromechanical materials, such as electroactive polymers, that are be optimized for applications like artificial muscles made of chainlike molecules that change in size ...
... aligned carbon-nanotube electrodes can enhance ion performance in an actuator, meaning they could Both devices typically contain electromechanical materials, such as electroactive polymers, that are be optimized for applications like artificial muscles made of chainlike molecules that change in size ...
Objective 1: Summarize the development of atomic theory
... 3. What is the frequency of radiation whose wavelength is 10.0 Angstroms? ...
... 3. What is the frequency of radiation whose wavelength is 10.0 Angstroms? ...
BL Web - The Bioluminescence Web Page
... that you are likely to encounter are non-luminous, but there is one deep-sea species which puts off a sparkling display of luminescence when disturbed. The main planktonic invertebrates (as distinct from protists) that don’t luminesce are the heteropods and pteropods (although many other molluscs ca ...
... that you are likely to encounter are non-luminous, but there is one deep-sea species which puts off a sparkling display of luminescence when disturbed. The main planktonic invertebrates (as distinct from protists) that don’t luminesce are the heteropods and pteropods (although many other molluscs ca ...
4) Spectroscopies Involving Energy Exchange
... (1) Fluorescence is generally observed with molecules where the lowest energy absorption is a π → π* transition, and those chromophores are called fluors or fluorephores. (2) For low concentrations of the fluorescing species, where εbC is less than 0.01, the intensity of fluorescence (If) is express ...
... (1) Fluorescence is generally observed with molecules where the lowest energy absorption is a π → π* transition, and those chromophores are called fluors or fluorephores. (2) For low concentrations of the fluorescing species, where εbC is less than 0.01, the intensity of fluorescence (If) is express ...
The excitation mechanism excitation mechanism
... with the gas molecules and transfer energy to them. Thus, the gas molecules are raised to excited state. Higher voltage is required to start the electrical discharge in the tube than to keep the discharge. Thus, a preliminary high voltage pulse is applied for initial discharge, and then the voltage ...
... with the gas molecules and transfer energy to them. Thus, the gas molecules are raised to excited state. Higher voltage is required to start the electrical discharge in the tube than to keep the discharge. Thus, a preliminary high voltage pulse is applied for initial discharge, and then the voltage ...
Wang_Project_Summery
... energy of all electron bunches inside the SPEAR ring. The Light Shack takes the synchrotron radiation from the SPEAR ring and filters out the harmful wavelength radiation with a cold finger, which is a piece of metal that blocks out x-ray radiations. As a result from the cold finger, only radiation ...
... energy of all electron bunches inside the SPEAR ring. The Light Shack takes the synchrotron radiation from the SPEAR ring and filters out the harmful wavelength radiation with a cold finger, which is a piece of metal that blocks out x-ray radiations. As a result from the cold finger, only radiation ...
Free-electron lasers
... (Synchrotron Light Source). This helps to make drug research more systematic and efficient. ...
... (Synchrotron Light Source). This helps to make drug research more systematic and efficient. ...
Mechanisms of Radio Wave Emission
... • Synchrotron Emission – Acceleration of charged particles by magnetic fields (learn more) – This can happen without excitation of the addition of heat. – Electrons spiral around the magnetic field and emit almost constant ...
... • Synchrotron Emission – Acceleration of charged particles by magnetic fields (learn more) – This can happen without excitation of the addition of heat. – Electrons spiral around the magnetic field and emit almost constant ...
Energy Vocabulary Foldable
... Chemical (ex: food), Mechanical (ex: sound and motion), Thermal (ex: heat), Nuclear (ex: fission or fusion of the nucleus of the atom), Electrical (ex: electricity) and Electromagnetic (ex: light and radio). 3. gasoline 4. light waves 5. water waves 6. sound waves 7. radio waves 8. energy in the foo ...
... Chemical (ex: food), Mechanical (ex: sound and motion), Thermal (ex: heat), Nuclear (ex: fission or fusion of the nucleus of the atom), Electrical (ex: electricity) and Electromagnetic (ex: light and radio). 3. gasoline 4. light waves 5. water waves 6. sound waves 7. radio waves 8. energy in the foo ...
1 EM Waves Intro
... The wave model of light fails when we shine light on zinc, which causes a release of photoelectrons. Increasing the intensity of the light does not always cause more electrons to be released, Emission depends on frequency (color) ...
... The wave model of light fails when we shine light on zinc, which causes a release of photoelectrons. Increasing the intensity of the light does not always cause more electrons to be released, Emission depends on frequency (color) ...
2.5 Bohr Model and Electron Energy
... The farther away from the nucleus the greater the Potential Energy for that electron. iv. The closer to the nucleus the less Potential Energy for that electron. b. Electrons can absorb energy (absorption) from their surroundings from another energy source, such as sunlight energy (A.K.A electromagne ...
... The farther away from the nucleus the greater the Potential Energy for that electron. iv. The closer to the nucleus the less Potential Energy for that electron. b. Electrons can absorb energy (absorption) from their surroundings from another energy source, such as sunlight energy (A.K.A electromagne ...
4/10/2006 Chapter 37 Lasers, a Model Atom and Zero Point Energy
... the same wavelength as the incoming photon, b) its direction is the same as the incoming photon, c) it is in phase with the incoming photon. Thus, it is coherent. This is the mechanism for creating laser light. ...
... the same wavelength as the incoming photon, b) its direction is the same as the incoming photon, c) it is in phase with the incoming photon. Thus, it is coherent. This is the mechanism for creating laser light. ...
Supplementary Notes on Volumetric Analysis
... (c) Based on the energies for the electronic transitions of the Balmer series, calculate the frequencies and wavelengths for the two lowest energy transitions of the Paschen series. ...
... (c) Based on the energies for the electronic transitions of the Balmer series, calculate the frequencies and wavelengths for the two lowest energy transitions of the Paschen series. ...