Ser-Ala-Trp
... peptide nitrogen atom is not free to rotate. D. The secondary structure affects the strength of the peptide unit. E. There is a large degree of freedom of rotation on either side of the peptide unit. ...
... peptide nitrogen atom is not free to rotate. D. The secondary structure affects the strength of the peptide unit. E. There is a large degree of freedom of rotation on either side of the peptide unit. ...
what are proteins? - scie
... therefore the function, of a protein depends entirely on the amino acid sequence. During digestion, proteins undergo hydrolysis and are split up into their component amino acids. The body can then use these as building blocks to make the proteins it needs. ...
... therefore the function, of a protein depends entirely on the amino acid sequence. During digestion, proteins undergo hydrolysis and are split up into their component amino acids. The body can then use these as building blocks to make the proteins it needs. ...
Amino acids, peptides and proteins
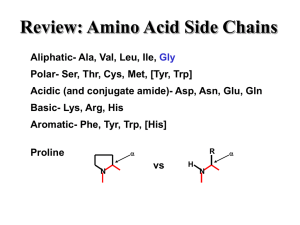
... Twenty different residues are involved in protein synthesis. These residues might be modified after the synthesis of the polypeptide chain. The other components of proteins are called prosthetic groups. The structure of the amino acids and their characteristic property as amphoteric molecules is des ...
... Twenty different residues are involved in protein synthesis. These residues might be modified after the synthesis of the polypeptide chain. The other components of proteins are called prosthetic groups. The structure of the amino acids and their characteristic property as amphoteric molecules is des ...
defend your answer in 1
... true If a hydrophobic amino acid found in a protein residing in the cytosol is replaced with an amino acid with a charged side group, the protein will likely turn inside out. 2. (3 pts.) Examine the diagram shown below which was taken from your textbook. Which sentence or sentences is(are) true with ...
... true If a hydrophobic amino acid found in a protein residing in the cytosol is replaced with an amino acid with a charged side group, the protein will likely turn inside out. 2. (3 pts.) Examine the diagram shown below which was taken from your textbook. Which sentence or sentences is(are) true with ...
Chapter 4 Problem Set
... for Peptide b. Because Peptide a has the lower value of ∆∆G˚, it is more likely to be folded into an helix. Note that ∆∆G˚ values are the differences in free energy change relative to alanine, that is required for an amino acid to take up the helical conformation. ...
... for Peptide b. Because Peptide a has the lower value of ∆∆G˚, it is more likely to be folded into an helix. Note that ∆∆G˚ values are the differences in free energy change relative to alanine, that is required for an amino acid to take up the helical conformation. ...
Biological Building Blocks Andrew Rylaarsdam
... After synthesizing and purifying these peptides, we were able to characterize them. First we verified that they were of the correct size and sequence, and second that they folded as we had hoped. With these peptides synthesized and characterized, we are beginning to study how they bind to metals, an ...
... After synthesizing and purifying these peptides, we were able to characterize them. First we verified that they were of the correct size and sequence, and second that they folded as we had hoped. With these peptides synthesized and characterized, we are beginning to study how they bind to metals, an ...
Why are Proteins Important in Organisms
... Interactions between the amino acids stabilize this shape, so that, like the telephone cord, it will automatically return to the shape after being stretched or bent. Pull your telephone cord out straight and let it snap back. Not only does it return to its helixshaped secondary structure, the helix ...
... Interactions between the amino acids stabilize this shape, so that, like the telephone cord, it will automatically return to the shape after being stretched or bent. Pull your telephone cord out straight and let it snap back. Not only does it return to its helixshaped secondary structure, the helix ...
Sports Fitness
... When you eat foods that contain protein, you break down the protein in food into basic units, called amino acids .The amino acids then can be reused to make the proteins your body needs to maintain muscles, bones, blood, and body organs. ...
... When you eat foods that contain protein, you break down the protein in food into basic units, called amino acids .The amino acids then can be reused to make the proteins your body needs to maintain muscles, bones, blood, and body organs. ...
Discovery of DNA... Bill Nye... https://youtu.be/VegLVn_1oCE The
... 2. mRNA moves out of the nucleus into cytoplasm then to a ribosome Ribosome: Organelle composed of RNA and protein. A ribosome uses mRNA, transfer RNA (tRNA), and amino acids to make proteins. 3. Translation happens at the ribosome. Every 3 mRNA bases (a codon) codes for 1 amino acid. mRNA codons pa ...
... 2. mRNA moves out of the nucleus into cytoplasm then to a ribosome Ribosome: Organelle composed of RNA and protein. A ribosome uses mRNA, transfer RNA (tRNA), and amino acids to make proteins. 3. Translation happens at the ribosome. Every 3 mRNA bases (a codon) codes for 1 amino acid. mRNA codons pa ...
peptides
... Oligopeptide (peptide): a short chain of 20-30 amino acids Polypeptide: a longer peptide with no particular structure Protein: a polypeptide chains with an organized 3D structures The average molecular weight of an amino acid residue is about 110 The molecular weights of most proteins are between 55 ...
... Oligopeptide (peptide): a short chain of 20-30 amino acids Polypeptide: a longer peptide with no particular structure Protein: a polypeptide chains with an organized 3D structures The average molecular weight of an amino acid residue is about 110 The molecular weights of most proteins are between 55 ...
Protein Structure
... Protein Overall Structure Primary (1ᵒ): Amino Acids in a chain/sequence Secondary (2ᵒ): Alpha helix or Beta pleated chain Tertiary (3ᵒ): Complex shape Quaternary (4ᵒ): 2 or more proteins folded on each other ...
... Protein Overall Structure Primary (1ᵒ): Amino Acids in a chain/sequence Secondary (2ᵒ): Alpha helix or Beta pleated chain Tertiary (3ᵒ): Complex shape Quaternary (4ᵒ): 2 or more proteins folded on each other ...
Introduction to Proteins
... been flopped over 90 degrees to the right for illustration purposes.) Cysteine has an R group that is similar but includes a sulfur atom. Other amino acids have R groups that range from a single hydrogen atom up to a double ring of carbon and nitrogen atoms, as you can see in the next plate. Color t ...
... been flopped over 90 degrees to the right for illustration purposes.) Cysteine has an R group that is similar but includes a sulfur atom. Other amino acids have R groups that range from a single hydrogen atom up to a double ring of carbon and nitrogen atoms, as you can see in the next plate. Color t ...
DN: Protein
... • proteins. True proteins are composed of long chains of amino acids, each protein distinguishable by its unique sequence of the 20 different amino acids as illustrated on the left. In the feed lab, protein is distinguishable from carbohydrate and lipid due to its content of nitrogen (N) feed protei ...
... • proteins. True proteins are composed of long chains of amino acids, each protein distinguishable by its unique sequence of the 20 different amino acids as illustrated on the left. In the feed lab, protein is distinguishable from carbohydrate and lipid due to its content of nitrogen (N) feed protei ...
Center for Structural Biology
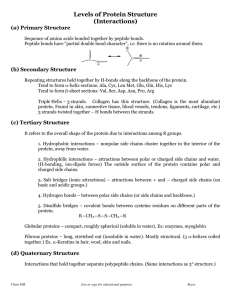
... Tertiary- organization of a complete chain Quaternary- organization of multiple chains ...
... Tertiary- organization of a complete chain Quaternary- organization of multiple chains ...
Protein structure
... Adjacent strands can be parallel or anti-parallel R-groups extend perpendicular to the plane of the H-bonds. R-groups of neighbouring residues within one -strand point in opposite ...
... Adjacent strands can be parallel or anti-parallel R-groups extend perpendicular to the plane of the H-bonds. R-groups of neighbouring residues within one -strand point in opposite ...
Maintaining Linkage: More examples
... Both HIFα and ARNT contain an N-terminal bHLH DNA binding domain and two adjacent PAS domains, referred to as PAS-A and PAS-B. PAS domains are structural modules found in proteins from all kingdoms of life that have significant structural homology despite little conservation of amino acid sequence. ...
... Both HIFα and ARNT contain an N-terminal bHLH DNA binding domain and two adjacent PAS domains, referred to as PAS-A and PAS-B. PAS domains are structural modules found in proteins from all kingdoms of life that have significant structural homology despite little conservation of amino acid sequence. ...
File
... • Amino acid sequences will commonly fold into two stable configurations, called secondary structures. • Alpha helices occur when the amino acid sequence folds into a coil/spiral arrangement • Beta-pleated sheets occur when the amino acid sequences adopts a directionally-oriented staggered strand co ...
... • Amino acid sequences will commonly fold into two stable configurations, called secondary structures. • Alpha helices occur when the amino acid sequence folds into a coil/spiral arrangement • Beta-pleated sheets occur when the amino acid sequences adopts a directionally-oriented staggered strand co ...
Amino acid sequence fingerprints in divergent evolution of
... In the case the proteins and/or enzymes form families with members related by their function (e.g., the members can differ by their detailed enzyme specificity), then it should be possible to identify the amino acid residues responsible for the subtle differences among the family members. This means ...
... In the case the proteins and/or enzymes form families with members related by their function (e.g., the members can differ by their detailed enzyme specificity), then it should be possible to identify the amino acid residues responsible for the subtle differences among the family members. This means ...
Relationship between amino acids sequences and protein structures
... regularities a new algorithm of the multiple sequences alignment was developed. The proposed method involves ‘projecting’ common supersecondary structural characteristics onto the sequence to reveal key residues. The results of the alignment revealed conserved positions. It was shown that residues a ...
... regularities a new algorithm of the multiple sequences alignment was developed. The proposed method involves ‘projecting’ common supersecondary structural characteristics onto the sequence to reveal key residues. The results of the alignment revealed conserved positions. It was shown that residues a ...
Protein Structure 2 - Interactions - Hydrolysis
... Repeating structures held together by H-bonds along the backbone of the protein. Tend to form α-helix sections: Ala, Cys, Leu Met, Glu, Gln, His, Lys Tend to form β-sheet sections: Val, Ser, Asp, Asn, Pro, Arg Triple Helix – 3 strands. Collagen has this structure. (Collagen is the most abundant prot ...
... Repeating structures held together by H-bonds along the backbone of the protein. Tend to form α-helix sections: Ala, Cys, Leu Met, Glu, Gln, His, Lys Tend to form β-sheet sections: Val, Ser, Asp, Asn, Pro, Arg Triple Helix – 3 strands. Collagen has this structure. (Collagen is the most abundant prot ...
Four Levels of Protein Structure
... Four Levels of Protein Structure • Primary Structure: Linear Sequence of Amino Acids Each amino acid has central carbon liked to ---hydrogen (H) ---amino group (NH2) ...
... Four Levels of Protein Structure • Primary Structure: Linear Sequence of Amino Acids Each amino acid has central carbon liked to ---hydrogen (H) ---amino group (NH2) ...