Chapter 16 - Faculty Server Contact
... temperature • The zeroth law states: If two systems A and B are both in thermal equilibrium with a third system, then A and B are in thermal equilibrium with each other • Based on the idea of thermal equilibrium • The zeroth law restatement: Suppose the temperature of system A is equal to the temper ...
... temperature • The zeroth law states: If two systems A and B are both in thermal equilibrium with a third system, then A and B are in thermal equilibrium with each other • Based on the idea of thermal equilibrium • The zeroth law restatement: Suppose the temperature of system A is equal to the temper ...
Unit II - Chemical Thermodynamics
... Reversible process: It is a process which takes place infinitesimally slowly so that the system is in thermodynamic equilibrium at any instant of the change. Since the process is carried out extremely slowly the properties of the system remain virtually unchanged and the direction may be reversed by ...
... Reversible process: It is a process which takes place infinitesimally slowly so that the system is in thermodynamic equilibrium at any instant of the change. Since the process is carried out extremely slowly the properties of the system remain virtually unchanged and the direction may be reversed by ...
The Helmholtz Function
... a fundamental set of four, all of which are energies and hence conserved. They are: U the internal energy H enthalpy ( old fashioned name is “heat content”) Important when discussing heat capacities and latent heats. F Helmholtz Function. Often A is used as the symbol. Important in statistical mecha ...
... a fundamental set of four, all of which are energies and hence conserved. They are: U the internal energy H enthalpy ( old fashioned name is “heat content”) Important when discussing heat capacities and latent heats. F Helmholtz Function. Often A is used as the symbol. Important in statistical mecha ...
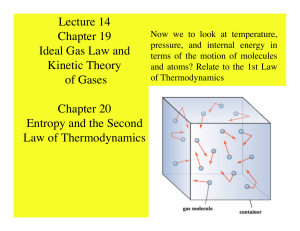
Lecture 14 Chapter 19 Ideal Gas Law and Kinetic Theory of Gases
... added to the gas while the volume was expanding or else it would have cooled. Since heat was added, Q is +, Hence, the entropy change was + or ΔS > 0 The change in entropy for a free expansion is also given by the above formula since the temperature doesn’t change as well. ...
... added to the gas while the volume was expanding or else it would have cooled. Since heat was added, Q is +, Hence, the entropy change was + or ΔS > 0 The change in entropy for a free expansion is also given by the above formula since the temperature doesn’t change as well. ...
Thermodynamic Wrap-up
... a. Identify, given a graph relating the quantity of heat added to a substance and its temperature, the melting point and boiling point and determine the heats of fusion and vaporization and the specific heat of each phase. This requires you to interpret a standard temperature Vs energy graph. Consul ...
... a. Identify, given a graph relating the quantity of heat added to a substance and its temperature, the melting point and boiling point and determine the heats of fusion and vaporization and the specific heat of each phase. This requires you to interpret a standard temperature Vs energy graph. Consul ...
An Empirical Formula of Mean Specific Heat Capacity of Ideal Gases
... Abstract. The method of formulation of tabular data of mean specific heat capacity of gases is discussed and an empirical formula to fit these data is given in this paper. A linear function of temperature is chosen as the formula to piecewise fit mean isobaric specific heat capacity data over a wide ...
... Abstract. The method of formulation of tabular data of mean specific heat capacity of gases is discussed and an empirical formula to fit these data is given in this paper. A linear function of temperature is chosen as the formula to piecewise fit mean isobaric specific heat capacity data over a wide ...
Chapter 5. Thermochemistry.
... If a reaction is carried out at constant P, which is true for all reactions open to the atmosphere, e.g. in a beaker, then the work done by the system is equal to -P ΔV. However, the change in volume of a solution will generally be very small, and so this can be ignored. The heat given off or absorb ...
... If a reaction is carried out at constant P, which is true for all reactions open to the atmosphere, e.g. in a beaker, then the work done by the system is equal to -P ΔV. However, the change in volume of a solution will generally be very small, and so this can be ignored. The heat given off or absorb ...
Thermodynamics - Atmosphere Physics
... The 1/2mv2 is the kinetic energy of a molecule of mass m moving with a velocity v. There is ½ kT of energy “per degree of freedom” of the molecule. For a molecule moving in 3-dimensions, there are 3 degrees of freedom and thus the average kinetic energy is stored as 3/2kT. If there are other ways fo ...
... The 1/2mv2 is the kinetic energy of a molecule of mass m moving with a velocity v. There is ½ kT of energy “per degree of freedom” of the molecule. For a molecule moving in 3-dimensions, there are 3 degrees of freedom and thus the average kinetic energy is stored as 3/2kT. If there are other ways fo ...
Equipartition theorem

In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions.The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every atom in a monatomic ideal gas has an average kinetic energy of (3/2)kBT in thermal equilibrium, where kB is the Boltzmann constant and T is the (thermodynamic) temperature. More generally, it can be applied to any classical system in thermal equilibrium, no matter how complicated. The equipartition theorem can be used to derive the ideal gas law, and the Dulong–Petit law for the specific heat capacities of solids. It can also be used to predict the properties of stars, even white dwarfs and neutron stars, since it holds even when relativistic effects are considered.Although the equipartition theorem makes very accurate predictions in certain conditions, it becomes inaccurate when quantum effects are significant, such as at low temperatures. When the thermal energy kBT is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be ""frozen out"" when the thermal energy is much smaller than this spacing. For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required. Along with other evidence, equipartition's failure to model black-body radiation—also known as the ultraviolet catastrophe—led Max Planck to suggest that energy in the oscillators in an object, which emit light, were quantized, a revolutionary hypothesis that spurred the development of quantum mechanics and quantum field theory.