I. Development of the Virial Theorem
... Thus far, with the exception of a brief discussion in Section 2, we have developed Lagrange's identity in a variety of ways, but have not rigorously taken that finial step to produce the virial theorem. This last step involves averaging over time and it is in this form that the theorem finds its wid ...
... Thus far, with the exception of a brief discussion in Section 2, we have developed Lagrange's identity in a variety of ways, but have not rigorously taken that finial step to produce the virial theorem. This last step involves averaging over time and it is in this form that the theorem finds its wid ...
I. NEWTONIAN MECHANICS
... (C) The velocities of both skaters will be equal. impulse delivered to the passenger. (D) The speeds of both skaters will be equal. (E) The impulse delivered to the passenger is reduced by the presence of the airbag. (E) The momenta of the two skaters are equal in magnitude. 1029. Two objects collid ...
... (C) The velocities of both skaters will be equal. impulse delivered to the passenger. (D) The speeds of both skaters will be equal. (E) The impulse delivered to the passenger is reduced by the presence of the airbag. (E) The momenta of the two skaters are equal in magnitude. 1029. Two objects collid ...
PPT
... There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of classical thermodynamics, entropy is defined as ...
... There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of classical thermodynamics, entropy is defined as ...
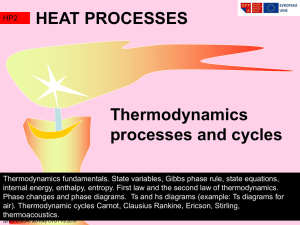
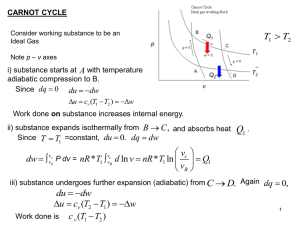
PC1221 Fundamentals of Physics I Ground Rules Thermodynamics
... Thermodynamics and mechanics were considered to be separate branches ...
... Thermodynamics and mechanics were considered to be separate branches ...
Document
... The enthalpy change taking place when one mol of solute is dissolved in one mol of solvent . Enthalpy of atomization(∆aH°): The enthalpy change taking place when one mol of solid or molecule or compound is changed into constituents atoms. ...
... The enthalpy change taking place when one mol of solute is dissolved in one mol of solvent . Enthalpy of atomization(∆aH°): The enthalpy change taking place when one mol of solid or molecule or compound is changed into constituents atoms. ...
Review of classical thermodynamics
... in the system). But there are too many particles (NA = 6.022×1023 mol-1) and this approach in unpractical in most cases. 2. Classical (continuum) thermodynamics – to describe the material in terms of average quantities, or thermodynamic variables, such as temperature, internal energy, pressure, etc. ...
... in the system). But there are too many particles (NA = 6.022×1023 mol-1) and this approach in unpractical in most cases. 2. Classical (continuum) thermodynamics – to describe the material in terms of average quantities, or thermodynamic variables, such as temperature, internal energy, pressure, etc. ...
Chapter 4: Energy Analysis of Closed Systems
... Specific Heats and Changes in Internal Energy and Enthalpy for Ideal Gases Before the first law of thermodynamics can be applied to systems, ways to calculate the change in internal energy of the substance enclosed by the system boundary must be determined. For real substances like water, the prope ...
... Specific Heats and Changes in Internal Energy and Enthalpy for Ideal Gases Before the first law of thermodynamics can be applied to systems, ways to calculate the change in internal energy of the substance enclosed by the system boundary must be determined. For real substances like water, the prope ...
Thermodynamics Notes
... another, but we would like to have a quantitative measure of temperature. A number of temperature scales have been devised, based on the temperature difference between two easily recognized conditions, such as the freezing and boiling of water. Beyond that, the definition of a degree of temperature ...
... another, but we would like to have a quantitative measure of temperature. A number of temperature scales have been devised, based on the temperature difference between two easily recognized conditions, such as the freezing and boiling of water. Beyond that, the definition of a degree of temperature ...
JIF 314 Thermodynamics - comsics
... A pot of containing water (system A) Boundary – the wall of the pot The surrounding (system B)– the atmosphere out side the pot at room temperature X, If initially the pot contains boiling water, the temperature (X) is not constant but will keep dropping. Hence during this period, both system A and ...
... A pot of containing water (system A) Boundary – the wall of the pot The surrounding (system B)– the atmosphere out side the pot at room temperature X, If initially the pot contains boiling water, the temperature (X) is not constant but will keep dropping. Hence during this period, both system A and ...
Section 3 Entropy and Classical Thermodynamics
... 3.1.1 The Second Law of Thermodynamics There are various statements of the second law of thermodynamics. These must obviously be logically equivalent. In the spirit of our approach we shall adopt the following statement: • There exists an extensive function of state called entropy, such that in any ...
... 3.1.1 The Second Law of Thermodynamics There are various statements of the second law of thermodynamics. These must obviously be logically equivalent. In the spirit of our approach we shall adopt the following statement: • There exists an extensive function of state called entropy, such that in any ...
Chapter 5 auxiliary functions
... * From the second law of thermodynamics : q ≤ T(S2 –S1) ≤ – ΔGَw therefore for reversible processes that occur at constant temperature and pressure ; the maximum amount of work , other than the p – v work is given by equation : max = – ΔGَw * again the pervious inequality can b written as ; = – (ΔG ...
... * From the second law of thermodynamics : q ≤ T(S2 –S1) ≤ – ΔGَw therefore for reversible processes that occur at constant temperature and pressure ; the maximum amount of work , other than the p – v work is given by equation : max = – ΔGَw * again the pervious inequality can b written as ; = – (ΔG ...
1 Thermodynamics Reference: Any physical chemistry text book See
... Nicholls and Ferguson Bioenergetics 2 . Chapter 3 Cramer and Knaff Energy transduction in biological membranes. Chapter 1-2 Voet and Voet Biochemistry Chapter 2-3 Also look in your favorite Biochemistry; Physical Chemistry, or Organic Chemistry Books for the sections on Thermodynamics & the section ...
... Nicholls and Ferguson Bioenergetics 2 . Chapter 3 Cramer and Knaff Energy transduction in biological membranes. Chapter 1-2 Voet and Voet Biochemistry Chapter 2-3 Also look in your favorite Biochemistry; Physical Chemistry, or Organic Chemistry Books for the sections on Thermodynamics & the section ...
Document
... collisions of molecules in the substance. • In convection, bulk quantities of the substance flow to areas of different temperature. • Radiation is the transfer of energy by ...
... collisions of molecules in the substance. • In convection, bulk quantities of the substance flow to areas of different temperature. • Radiation is the transfer of energy by ...
Powerpoints - University of Pittsburgh
... Only ONE equation is needed. Particles in suspension = a fixed, large number of component that do not interact with each other. Hence they can be treated by exactly the same analysis as an ideal gas and dilute sugar solution! The particles exert a pressure due to their thermal motions. ...
... Only ONE equation is needed. Particles in suspension = a fixed, large number of component that do not interact with each other. Hence they can be treated by exactly the same analysis as an ideal gas and dilute sugar solution! The particles exert a pressure due to their thermal motions. ...
Equipartition theorem

In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions.The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every atom in a monatomic ideal gas has an average kinetic energy of (3/2)kBT in thermal equilibrium, where kB is the Boltzmann constant and T is the (thermodynamic) temperature. More generally, it can be applied to any classical system in thermal equilibrium, no matter how complicated. The equipartition theorem can be used to derive the ideal gas law, and the Dulong–Petit law for the specific heat capacities of solids. It can also be used to predict the properties of stars, even white dwarfs and neutron stars, since it holds even when relativistic effects are considered.Although the equipartition theorem makes very accurate predictions in certain conditions, it becomes inaccurate when quantum effects are significant, such as at low temperatures. When the thermal energy kBT is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be ""frozen out"" when the thermal energy is much smaller than this spacing. For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required. Along with other evidence, equipartition's failure to model black-body radiation—also known as the ultraviolet catastrophe—led Max Planck to suggest that energy in the oscillators in an object, which emit light, were quantized, a revolutionary hypothesis that spurred the development of quantum mechanics and quantum field theory.


















![Assemblage: Exercises in Statistical Mechanics ====== [A] Ensemble Theory - classical gases](http://s1.studyres.com/store/data/008930189_1-a7a37d9ca413714c6a603f524253db38-300x300.png)
