T - Himastron
... a system (an “expansion” work) - PdV and all other kinds of work, Wother (electrical work, work on creating the surface area, etc.): ...
... a system (an “expansion” work) - PdV and all other kinds of work, Wother (electrical work, work on creating the surface area, etc.): ...
Chapter 6 NOTES!!!!! - Clinton Public Schools
... Heat Conductors • Collisions between these electrons and other particles in the metal enable thermal energy to be transferred more quickly than in other material. ...
... Heat Conductors • Collisions between these electrons and other particles in the metal enable thermal energy to be transferred more quickly than in other material. ...
[cond-mat.stat-mech] 29 Jul 1999 - Data Analysis and Modeling of
... In current usage, the terms “microscopically reversible” and “detailed balance” are often used interchangeably [26]. However, the original meaning of microscopic reversibility [27,28] is similar to Eq. (5). It relates the probability of a particular path to its reverse. This is distinct from the pri ...
... In current usage, the terms “microscopically reversible” and “detailed balance” are often used interchangeably [26]. However, the original meaning of microscopic reversibility [27,28] is similar to Eq. (5). It relates the probability of a particular path to its reverse. This is distinct from the pri ...
Exercises in Statistical Mechanics (2004)
... 001 Random variables (distance between two particles in a box) 002 Random variables (length of chain molecule) 003 Random variables (fluctuations in number of particles) 005 Random variable x=cos(theta) 006 Microcanonical state of oscillator 007 The ergodic density rho(x) 008 Spreading of free parti ...
... 001 Random variables (distance between two particles in a box) 002 Random variables (length of chain molecule) 003 Random variables (fluctuations in number of particles) 005 Random variable x=cos(theta) 006 Microcanonical state of oscillator 007 The ergodic density rho(x) 008 Spreading of free parti ...
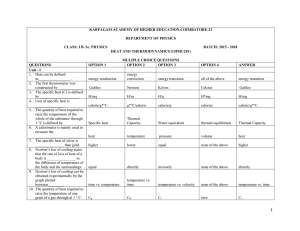
3. JEE-main Physics 2015
... is equal to : (g = gravitational acceleration) T 2 A M (1) T 1 Mg ...
... is equal to : (g = gravitational acceleration) T 2 A M (1) T 1 Mg ...
253 Chapter 12 Thermodynamics GOALS When you have mastered
... internal energy of a system depends only on the state of the system. For this reason it is called a state function. A state function is dependent only on the variables defining the state of the system such as the pressure, temperature, and volume for an ideal gas. A state function is independent of ...
... internal energy of a system depends only on the state of the system. For this reason it is called a state function. A state function is dependent only on the variables defining the state of the system such as the pressure, temperature, and volume for an ideal gas. A state function is independent of ...
GHW#12-Chapter-6-Tro
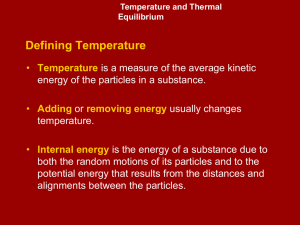
... Measuring thermal energy changes Thermal energy cannot be directly measured. We can only measure differences in energy. To be able to observe energy changes, we must be able to isolate our system from the rest of the universe. Calorimeter - a device that is used to measure thermal energy changes an ...
... Measuring thermal energy changes Thermal energy cannot be directly measured. We can only measure differences in energy. To be able to observe energy changes, we must be able to isolate our system from the rest of the universe. Calorimeter - a device that is used to measure thermal energy changes an ...
Second Law of Thermodynamics
... Again, we can use (4.4) (Tds = du + pdα, in reversible form) to obtain dg = -sdT + αdp. In this case, if T and p are constant, for a body in equilibrium we have dg = 0. For an irreversible process, dg < -sdT + αdp. Thus, dg < 0 in an irreversible, isobaric, isothermal transformation. Gibbs free ener ...
... Again, we can use (4.4) (Tds = du + pdα, in reversible form) to obtain dg = -sdT + αdp. In this case, if T and p are constant, for a body in equilibrium we have dg = 0. For an irreversible process, dg < -sdT + αdp. Thus, dg < 0 in an irreversible, isobaric, isothermal transformation. Gibbs free ener ...
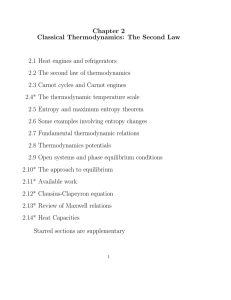
Chapter 2 Classical Thermodynamics: The Second Law 2.1 Heat
... Do you know how a real refrigerator works? What is the common working substance used? Can efficiency of refrigerators as defined in Eq. (10) be greater than ...
... Do you know how a real refrigerator works? What is the common working substance used? Can efficiency of refrigerators as defined in Eq. (10) be greater than ...
Equipartition theorem

In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions.The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every atom in a monatomic ideal gas has an average kinetic energy of (3/2)kBT in thermal equilibrium, where kB is the Boltzmann constant and T is the (thermodynamic) temperature. More generally, it can be applied to any classical system in thermal equilibrium, no matter how complicated. The equipartition theorem can be used to derive the ideal gas law, and the Dulong–Petit law for the specific heat capacities of solids. It can also be used to predict the properties of stars, even white dwarfs and neutron stars, since it holds even when relativistic effects are considered.Although the equipartition theorem makes very accurate predictions in certain conditions, it becomes inaccurate when quantum effects are significant, such as at low temperatures. When the thermal energy kBT is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be ""frozen out"" when the thermal energy is much smaller than this spacing. For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required. Along with other evidence, equipartition's failure to model black-body radiation—also known as the ultraviolet catastrophe—led Max Planck to suggest that energy in the oscillators in an object, which emit light, were quantized, a revolutionary hypothesis that spurred the development of quantum mechanics and quantum field theory.








![[cond-mat.stat-mech] 29 Jul 1999 - Data Analysis and Modeling of](http://s1.studyres.com/store/data/004609137_1-3d6203405239cf93abc08201b80fbc47-300x300.png)