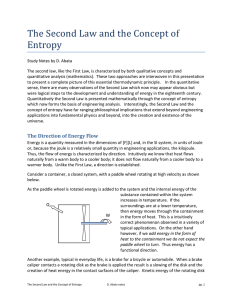
Chapter 6 NOTES!!!!! - Clinton Public Schools
... in joulesthe same units that energy is measured in. • Heat always flows from warmer to cooler materials. ...
... in joulesthe same units that energy is measured in. • Heat always flows from warmer to cooler materials. ...
Heat Engines and the First Law of Thermodynamics
... it. Observations like these caused physicists and engineers in the middle of the nineteenth century to conclude that heat is just a form of energy, the form that flows when there is a temperature difference between two objects. We want to examine the mathematical relationship between the heat energy ...
... it. Observations like these caused physicists and engineers in the middle of the nineteenth century to conclude that heat is just a form of energy, the form that flows when there is a temperature difference between two objects. We want to examine the mathematical relationship between the heat energy ...
File
... and pd. The equilibrium pressures are PA,PB,PC and PD. The following theoretical operations are performed. 1) Change of pressure of A from pa to PA work done by the gas = RT ln pa / PA 2) Change of pressure of B from pb to PB work done by the gas = RT ln pb / PB 3) Now introduce 1 mole of A and B at ...
... and pd. The equilibrium pressures are PA,PB,PC and PD. The following theoretical operations are performed. 1) Change of pressure of A from pa to PA work done by the gas = RT ln pa / PA 2) Change of pressure of B from pb to PB work done by the gas = RT ln pb / PB 3) Now introduce 1 mole of A and B at ...
thermodynamics
... C is known as molar specific heat capacity of the substance. Like s, C is independent of the amount of substance. C depends on the nature of the substance, its temperature and the conditions under which heat is supplied. The unit of C is J mo1–1 K–1. As we shall see later (in connection with specifi ...
... C is known as molar specific heat capacity of the substance. Like s, C is independent of the amount of substance. C depends on the nature of the substance, its temperature and the conditions under which heat is supplied. The unit of C is J mo1–1 K–1. As we shall see later (in connection with specifi ...
Calorimetry Measurement
... atoms within the molecules. The zeroeth law of thermodynamics states that, if two systems are each in equilibrium with a third system, they will be in equilibrium with each other. They are said to have the same temperature. For gases, statistical mechanics shows the direct relation between the therm ...
... atoms within the molecules. The zeroeth law of thermodynamics states that, if two systems are each in equilibrium with a third system, they will be in equilibrium with each other. They are said to have the same temperature. For gases, statistical mechanics shows the direct relation between the therm ...
Thermodynamic temperature
... scale for thermodynamic temperature. It uses the Kelvin possible to complete rest; that is, they have minimal moscale for measurement and selects the triple point of water tion, retaining only quantum mechanical motion.[3] Zero at 273.16K as the fundamental fixing point. Other scales kinetic energy r ...
... scale for thermodynamic temperature. It uses the Kelvin possible to complete rest; that is, they have minimal moscale for measurement and selects the triple point of water tion, retaining only quantum mechanical motion.[3] Zero at 273.16K as the fundamental fixing point. Other scales kinetic energy r ...
The Fluctuation-imposed Limit for Temperature Measurement
... is to be measured, is assumed to be isothermal and to have infinite heat capacity. The thermometer incorporates an easily measured quantity, like the magnetization of a salt, whose mean value is a function of temperature and can thus be used to infer the reservoir temperature. From statistical mecha ...
... is to be measured, is assumed to be isothermal and to have infinite heat capacity. The thermometer incorporates an easily measured quantity, like the magnetization of a salt, whose mean value is a function of temperature and can thus be used to infer the reservoir temperature. From statistical mecha ...
about a variety of material equilibrium conditions
... In open systems entropy S and volume V changes with necessity at change of common number of moles of system N (by mass transfer), and also at change of its composition (by diffusion). That circumstance breaks a condition of its constancy, underlying in above mentioned definition of chemical potentia ...
... In open systems entropy S and volume V changes with necessity at change of common number of moles of system N (by mass transfer), and also at change of its composition (by diffusion). That circumstance breaks a condition of its constancy, underlying in above mentioned definition of chemical potentia ...
Elementary Notes on Classical Thermodynamics
... in the definition of heat measuring unit, is an important quantity in Thermodynamics. In general, the heat capacity (or thermal capacity) of a body is, by definition, the ratio (δQ/dT ) between an infinitesimal quantity of heat absorbed by the body and the related, infinitesimal increase of its temp ...
... in the definition of heat measuring unit, is an important quantity in Thermodynamics. In general, the heat capacity (or thermal capacity) of a body is, by definition, the ratio (δQ/dT ) between an infinitesimal quantity of heat absorbed by the body and the related, infinitesimal increase of its temp ...
Heat Effects - Association of Chemical Engineering Students
... is to develop relations between the quantity of heat transferred and the resulting temperature change. When the system is a homogeneous substance of constant composition, the phase rule indicates that fixing the values of two intensive properties establishes its state. The molar or specific internal ...
... is to develop relations between the quantity of heat transferred and the resulting temperature change. When the system is a homogeneous substance of constant composition, the phase rule indicates that fixing the values of two intensive properties establishes its state. The molar or specific internal ...
Chapter 27 - Houston ISD
... the bottom of the pot rises to the top of the pot, replaced by the cooler water. Next the cooler water is heated. If this did not happen, we would have to rely on the slower method of conduction to boil a pot of water. Why wearing a Through the process of convection, air carries heat away from your ...
... the bottom of the pot rises to the top of the pot, replaced by the cooler water. Next the cooler water is heated. If this did not happen, we would have to rely on the slower method of conduction to boil a pot of water. Why wearing a Through the process of convection, air carries heat away from your ...
chapter 3 thermodynamics of dilute gases
... All these results come from the functional form (3.26) in which the entropy depends on space and time implicitly through the functions h ( x, y, z, t ) and P ( x, y, z, t ) . The entropy does not depend explicitly on x, y, z, or t . Take the substantial derivative, D Dt , of the entropy. The resul ...
... All these results come from the functional form (3.26) in which the entropy depends on space and time implicitly through the functions h ( x, y, z, t ) and P ( x, y, z, t ) . The entropy does not depend explicitly on x, y, z, or t . Take the substantial derivative, D Dt , of the entropy. The resul ...
heat and temperature
... During the transition from one physical state to another, heat, the energy absorbed by a body is not used to increase the agitation (measured by the temperature) of its molecules, but in breaking the bonding among them. ...
... During the transition from one physical state to another, heat, the energy absorbed by a body is not used to increase the agitation (measured by the temperature) of its molecules, but in breaking the bonding among them. ...
q 2 - q 1
... Now if we consider the condensation process, i.e. if the cyclic process started by increasing the external pressure, which equals PH2O(T), by Δ P, it is possible to show that, for a cycle, the permanent change in the external energy of the heat reservoir is V ΔP. Thus, as ΔP approaches infinitesi ...
... Now if we consider the condensation process, i.e. if the cyclic process started by increasing the external pressure, which equals PH2O(T), by Δ P, it is possible to show that, for a cycle, the permanent change in the external energy of the heat reservoir is V ΔP. Thus, as ΔP approaches infinitesi ...