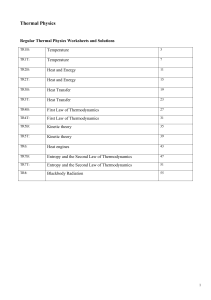
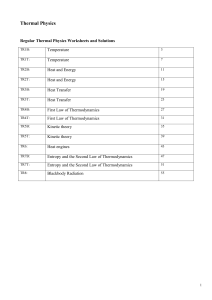
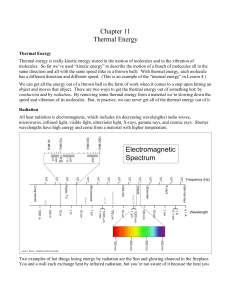
Atomic Structure
... Can you think of a use for such a strip? C. Quantitative Questions: 1. Aluminium rivets used in aeroplane construction are made slightly larger than the rivet holes and cooled by dry ice (solid carbon dioxide) before being driven in. If the diameter of a hole is 0.3000 cm, what should be the diamete ...
... Can you think of a use for such a strip? C. Quantitative Questions: 1. Aluminium rivets used in aeroplane construction are made slightly larger than the rivet holes and cooled by dry ice (solid carbon dioxide) before being driven in. If the diameter of a hole is 0.3000 cm, what should be the diamete ...
Module P7.3 Internal energy, heat and energy transfer
... The systems that are studied in thermodynamics are generally ones that can exist in several different states, each of which is specified by the values of various macroscopic properties of the system. For example, a fixed quantity of air trapped in a bicycle pump might be at a high temperature or a ...
... The systems that are studied in thermodynamics are generally ones that can exist in several different states, each of which is specified by the values of various macroscopic properties of the system. For example, a fixed quantity of air trapped in a bicycle pump might be at a high temperature or a ...
UNITS AND DIMENSIONS
... The most accurate way to determine the physical properties of foods is to determine the physical property experimentally. However, the physical properties of foods can also be determined by using equations developed for this purpose. Some of these equations were given in below. In literature, the ph ...
... The most accurate way to determine the physical properties of foods is to determine the physical property experimentally. However, the physical properties of foods can also be determined by using equations developed for this purpose. Some of these equations were given in below. In literature, the ph ...
Pdf - Text of NPTEL IIT Video Lectures
... equal to constant. Then 2 to 3 that is the constant pressure process where heat is added here at constant pressure process so 2 to 3 is a constant pressure process. Then 3 to 4 again an isentropic expansion and this is the direction 4to 1 is again the constant pressure heat rejection in a close cycl ...
... equal to constant. Then 2 to 3 that is the constant pressure process where heat is added here at constant pressure process so 2 to 3 is a constant pressure process. Then 3 to 4 again an isentropic expansion and this is the direction 4to 1 is again the constant pressure heat rejection in a close cycl ...
Unit 1 Mole and enthalpy changes
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
... Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change in enthalpy, the ...
Ch 15) The Laws of Thermodynamics
... law of thermodynamics is a general statement of the law of conservation of energy. Note that the conservation of energy law was not able to be formulated until the 1800s, because it depended on the interpretation of heat as a transfer of energy. A given system does not “have” a certain amount of hea ...
... law of thermodynamics is a general statement of the law of conservation of energy. Note that the conservation of energy law was not able to be formulated until the 1800s, because it depended on the interpretation of heat as a transfer of energy. A given system does not “have” a certain amount of hea ...
Chapter 4
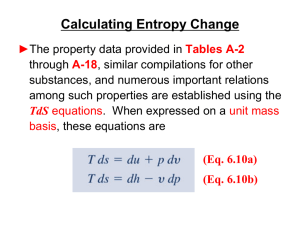
... Calculating Entropy Change of an Ideal Gas Example: Air undergoes a process from T1 = 620 K, p1 = 12 bar to a final state where s2 = s1, p2 = 1.4 bar. Employing the ideal gas model, determine the final temperature T2, in K. Solve using (a) pr data from Table A-22 and (b) a constant specific heat ra ...
... Calculating Entropy Change of an Ideal Gas Example: Air undergoes a process from T1 = 620 K, p1 = 12 bar to a final state where s2 = s1, p2 = 1.4 bar. Employing the ideal gas model, determine the final temperature T2, in K. Solve using (a) pr data from Table A-22 and (b) a constant specific heat ra ...
The Kinetic Theory of Gases
... same for all paths. The work W done on the gas (the negative of the area under the curves) is different for each path. Therefore, from the first law of thermodynamics, the heat associated with a given change in temperature does not have a unique value as discussed in Section 20.4. We can address thi ...
... same for all paths. The work W done on the gas (the negative of the area under the curves) is different for each path. Therefore, from the first law of thermodynamics, the heat associated with a given change in temperature does not have a unique value as discussed in Section 20.4. We can address thi ...
Synthesis of NiMn2O4 assisted by high
... time is the same for all the cases, differences among the products as a function of the milling time are useful for evidencing that the mechanical work energy is being stored, at least partially, by the powder system. A low temperature (500 oC) is known to be insufficient for producing synthesis in ...
... time is the same for all the cases, differences among the products as a function of the milling time are useful for evidencing that the mechanical work energy is being stored, at least partially, by the powder system. A low temperature (500 oC) is known to be insufficient for producing synthesis in ...
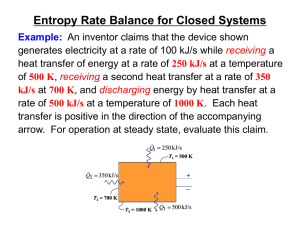
ch06C-2013
... The claim is in accord with the first law of thermodynamics. ► Applying an entropy rate balance dS 0 ...
... The claim is in accord with the first law of thermodynamics. ► Applying an entropy rate balance dS 0 ...
The high temperature heat capacity and related thermodynamic
... with two and so forth up to lutecium with a filled orbital containing 14 4f electrons. ...
... with two and so forth up to lutecium with a filled orbital containing 14 4f electrons. ...
Why is S(H2O(l) > S(H20(g)? It is better to speak of entropy as a
... Why is S(H2O(l) > S(H20(g)? It is better to speak of entropy as a measure of the amount of energy in a system that cannot be used to do work rather than an overly simplistic "measure of disorder". Recall that the units of entropy in the SI system are Joules/Kelvin (the units of heat capacity). From ...
... Why is S(H2O(l) > S(H20(g)? It is better to speak of entropy as a measure of the amount of energy in a system that cannot be used to do work rather than an overly simplistic "measure of disorder". Recall that the units of entropy in the SI system are Joules/Kelvin (the units of heat capacity). From ...