Chapter 19 Chemical Thermodynamics
... St. Charles Community College St. Peters, MO 2006, Prentice Hall, Inc. Modified by S.A. Green, 2006 ...
... St. Charles Community College St. Peters, MO 2006, Prentice Hall, Inc. Modified by S.A. Green, 2006 ...
AP Chemistry Study Guide 6 Evaporation vs. condensation
... Ø Change in Hcrystalization = -‐change in Hfusion Ø Generally much less than change in Hvap Ø Change in Hsublimination = change in Hfusion + change Hvaporization First law of thermodynamics Ø ...
... Ø Change in Hcrystalization = -‐change in Hfusion Ø Generally much less than change in Hvap Ø Change in Hsublimination = change in Hfusion + change Hvaporization First law of thermodynamics Ø ...
Exergy - SABİS
... A frictionless piston–cylinder device, shown in figure, initially contains 0.01 m3 of argon gas at 400 K and 350 kPa. Heat is now transferred to the argon from a furnace at 1200 K, and the argon expands isothermally until its volume is doubled. No heat transfer takes place between the argon and the ...
... A frictionless piston–cylinder device, shown in figure, initially contains 0.01 m3 of argon gas at 400 K and 350 kPa. Heat is now transferred to the argon from a furnace at 1200 K, and the argon expands isothermally until its volume is doubled. No heat transfer takes place between the argon and the ...
Meaning of Entropy in Classical Thermodynamics
... as a subject with an unusually high ratio of words to equations. In modern thermodynamics textbooks, this difficulty is circumvented by defining reversible, internally reversible, and externally or fully reversible processes [12,13]. Uffink, in his criticism of the liberal extrapolation of the Secon ...
... as a subject with an unusually high ratio of words to equations. In modern thermodynamics textbooks, this difficulty is circumvented by defining reversible, internally reversible, and externally or fully reversible processes [12,13]. Uffink, in his criticism of the liberal extrapolation of the Secon ...
Set 1 Answers
... 1. Explain the distinguishing properties of water and their chemical basis. Water has a high dielectric constant, high specific heat, high surface tension, and is less dense in the frozen state than the liquid state. The high surface tension is a result of hydrogen bonding which is very strong in wa ...
... 1. Explain the distinguishing properties of water and their chemical basis. Water has a high dielectric constant, high specific heat, high surface tension, and is less dense in the frozen state than the liquid state. The high surface tension is a result of hydrogen bonding which is very strong in wa ...
notes on thermodynamic formalism
... go temporarily out of equilibrium, at which point it may not have a well-defined pressure or temperature (for example). However, some properties of the system — for example, the total internal energy U — make sense even for a system out of equilibrium. More abstractly, we can imagine that the collec ...
... go temporarily out of equilibrium, at which point it may not have a well-defined pressure or temperature (for example). However, some properties of the system — for example, the total internal energy U — make sense even for a system out of equilibrium. More abstractly, we can imagine that the collec ...
ch06C-2013
... ► Since the rate of entropy production cannot be negative, the only compressor exit states that can be attained in an adiabatic compression are those with s2 ≥ s1. This is shown on the Mollier diagram to the right. ► The state labeled 2s on the figure would be attained only in an isentropic compress ...
... ► Since the rate of entropy production cannot be negative, the only compressor exit states that can be attained in an adiabatic compression are those with s2 ≥ s1. This is shown on the Mollier diagram to the right. ► The state labeled 2s on the figure would be attained only in an isentropic compress ...
S - BEHS Science
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
... First Law of Thermodynamics • You will recall from Chapter 5 that energy cannot be created nor destroyed. • Therefore, the total energy of the universe is a constant. • Energy can, however, be converted from one form to another or transferred from a system to the surroundings or vice versa. Chemica ...
Heat and Thermodynamics 300 MCQ
... 18. Which surface is the best radiator of heat 19. On a cloudiness night, the earth is gold because its heat is 20. The dimensional formula of K is 21. Thermal capacity of a good conductor is determined by 22. Thermal conductivity of a bad conductor is determined by 23. The SI unit of thermal conduc ...
... 18. Which surface is the best radiator of heat 19. On a cloudiness night, the earth is gold because its heat is 20. The dimensional formula of K is 21. Thermal capacity of a good conductor is determined by 22. Thermal conductivity of a bad conductor is determined by 23. The SI unit of thermal conduc ...
ME12001 Thermodynamics T7
... 1. Carefully define what the thermodynamic system is. 2. For multi-step processes with more than one step, identify the initial and final states for each step. 3. Identify the known quantities and the target variables. 4. The first law, ΔU = Q − W , can be applied just once to each step in a thermod ...
... 1. Carefully define what the thermodynamic system is. 2. For multi-step processes with more than one step, identify the initial and final states for each step. 3. Identify the known quantities and the target variables. 4. The first law, ΔU = Q − W , can be applied just once to each step in a thermod ...
Thermodynamic temperature
... motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., nonmechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational mo ...
... motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., nonmechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational mo ...
energy and power - Beck-Shop
... The heat energy contained within a body varies directly with its temperature, as implied in (1.19). The process of cooling implies a reduction of energy. Heat always flows spontaneously from a body of higher temperature to a body of lower temperature in an attempt to obtain a thermal energy equilibr ...
... The heat energy contained within a body varies directly with its temperature, as implied in (1.19). The process of cooling implies a reduction of energy. Heat always flows spontaneously from a body of higher temperature to a body of lower temperature in an attempt to obtain a thermal energy equilibr ...
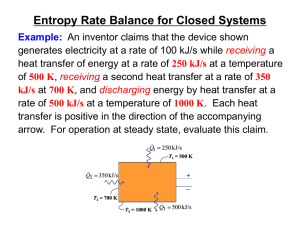
Second Law of Thermodynamics
... many practical applications. For example it explains the limits of efficiency for heat engines and refrigerators. To develop a better understanding of this law, try these conceptual questions. ...
... many practical applications. For example it explains the limits of efficiency for heat engines and refrigerators. To develop a better understanding of this law, try these conceptual questions. ...
Thermodynamics with Chemical Engineering Applications
... First introduction of the Helmholtz and Gibbs free energy functions. First and Second Laws combined in four versions 8.3 Dependence of S, U, H, A, and G on T, p, and V. Maxwell’s relations 8.3.1 Entropy vs. p–V–T 8.3.2 Internal energy vs. p–V –T 8.3.3 Enthalpy vs. p–V –T 8.3.4 Helmholtz free energy ...
... First introduction of the Helmholtz and Gibbs free energy functions. First and Second Laws combined in four versions 8.3 Dependence of S, U, H, A, and G on T, p, and V. Maxwell’s relations 8.3.1 Entropy vs. p–V–T 8.3.2 Internal energy vs. p–V –T 8.3.3 Enthalpy vs. p–V –T 8.3.4 Helmholtz free energy ...