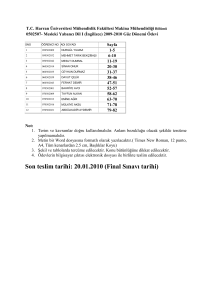
ee11042602mpt3.mov 110426ph423main3.mov Example of the
... system. That seems to be a theme behind several of the different extended comments that David had made, where students only keep track of what they as experimentalists change and not changes in the system as a result of other parts of the experiment. David noted that the student stated that this was ...
... system. That seems to be a theme behind several of the different extended comments that David had made, where students only keep track of what they as experimentalists change and not changes in the system as a result of other parts of the experiment. David noted that the student stated that this was ...
Physics 231 Topic 14: Laws of Thermodynamics Wade Fisher
... A New Powerplant A new powerplant is designed that makes use of the temperature difference between sea water at 0m (200) and at 1-km depth (50). A) what would be the maximum efficiency of such a plant? B) If the powerplant produces 75 MW, how much energy is absorbed per hour? C) Is this a good idea ...
... A New Powerplant A new powerplant is designed that makes use of the temperature difference between sea water at 0m (200) and at 1-km depth (50). A) what would be the maximum efficiency of such a plant? B) If the powerplant produces 75 MW, how much energy is absorbed per hour? C) Is this a good idea ...
Physics 231 Topic 14: Laws of Thermodynamics Wade Fisher
... A) Use U = 3/2nRT and PV=nRT so U=3/2PV & U=3/2(PV) U = 3/2(PfVf - PiVi) = 3/2[(6E+05)x1 - (3E+05)x4] = -9E+5 J B) Work: area under the P-V graph: (9+4.5)x105 = 13.5x105 (positive since work is done on the gas) C) U = Q+W so Q = U-W = (-9E+5)-(13.5E+5) = -22.5E+5 J Heat has been extracted from ...
... A) Use U = 3/2nRT and PV=nRT so U=3/2PV & U=3/2(PV) U = 3/2(PfVf - PiVi) = 3/2[(6E+05)x1 - (3E+05)x4] = -9E+5 J B) Work: area under the P-V graph: (9+4.5)x105 = 13.5x105 (positive since work is done on the gas) C) U = Q+W so Q = U-W = (-9E+5)-(13.5E+5) = -22.5E+5 J Heat has been extracted from ...
Vann - Chemistry ch. 6.1
... Suppose that a person exerts a force on the wheelbarrow that is initially at rest, causing it to move over a certain distance. Recall that the work done on the wheelbarrow by the person is equal to the product of the person's force multiplied by the distance traveled by the wheelbarrow. Notice that ...
... Suppose that a person exerts a force on the wheelbarrow that is initially at rest, causing it to move over a certain distance. Recall that the work done on the wheelbarrow by the person is equal to the product of the person's force multiplied by the distance traveled by the wheelbarrow. Notice that ...
Document
... between the system and surroundings Heat exchange occurs when system and surroundings have a difference in temperature Temperature is the measure of the amount of thermal energy within a sample of matter Heat flows from matter with high temperature to matter with low temperature until both objects r ...
... between the system and surroundings Heat exchange occurs when system and surroundings have a difference in temperature Temperature is the measure of the amount of thermal energy within a sample of matter Heat flows from matter with high temperature to matter with low temperature until both objects r ...
Document
... According to the first law of thermodynamics, heat and work are related through the “internal energy” of a system and generally cannot be interconverted. However, we can ask the question: How many times does a person need to lift a 500 N barbell a height of 2 m to correspond to 2000 Calories (1 Calo ...
... According to the first law of thermodynamics, heat and work are related through the “internal energy” of a system and generally cannot be interconverted. However, we can ask the question: How many times does a person need to lift a 500 N barbell a height of 2 m to correspond to 2000 Calories (1 Calo ...
Energy – conversion, conservation and management 1 Hellenistic
... only, this part of philosophy is no longer being developed Heller [6]. However in an implicit manner, by the creation of the paradigms of thinking, metaphysics is still present in the process of human cognition. For example, the thesis of quantized action, has its hidden implications in the modern q ...
... only, this part of philosophy is no longer being developed Heller [6]. However in an implicit manner, by the creation of the paradigms of thinking, metaphysics is still present in the process of human cognition. For example, the thesis of quantized action, has its hidden implications in the modern q ...
Energy – conversion, conservation and management 1 Hellenistic
... only, this part of philosophy is no longer being developed Heller [6]. However in an implicit manner, by the creation of the paradigms of thinking, metaphysics is still present in the process of human cognition. For example, the thesis of quantized action, has its hidden implications in the modern q ...
... only, this part of philosophy is no longer being developed Heller [6]. However in an implicit manner, by the creation of the paradigms of thinking, metaphysics is still present in the process of human cognition. For example, the thesis of quantized action, has its hidden implications in the modern q ...
Chapter 1: Introductory Concepts, Units, and Definitions
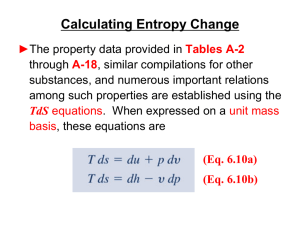
... or destroyed, but can only change its form. The three forms of energy storage of greatest interest to us are Potential Energy (PE), Kinetic Energy (KE), and Internal Energy (U), which we introduce below. The two forms of energy in transit that we consider are Work (W) and Heat (Q), and the interacti ...
... or destroyed, but can only change its form. The three forms of energy storage of greatest interest to us are Potential Energy (PE), Kinetic Energy (KE), and Internal Energy (U), which we introduce below. The two forms of energy in transit that we consider are Work (W) and Heat (Q), and the interacti ...
thermodynamics type 1
... Chemical reactions are generally carried out at constant pressure (atmospheric pressure) so it has been found useful to define a new state function Enthalpy (H) as : H = E + PV (By definition) or ∆H = ∆E + P ∆V + V∆P or ∆H = ∆E + P ∆V (at constant pressure) combining with first law. Equation (1) bec ...
... Chemical reactions are generally carried out at constant pressure (atmospheric pressure) so it has been found useful to define a new state function Enthalpy (H) as : H = E + PV (By definition) or ∆H = ∆E + P ∆V + V∆P or ∆H = ∆E + P ∆V (at constant pressure) combining with first law. Equation (1) bec ...
Thermodynamics and Kinetics
... part of the second law of thermodynamics: the entropy of the universe remains constant in a reversible process, whereas the entropy of the universe increases in an irreversible (spontaneous) process. Entropy is a state function described by the equation: where k is a proportionality constant equal t ...
... part of the second law of thermodynamics: the entropy of the universe remains constant in a reversible process, whereas the entropy of the universe increases in an irreversible (spontaneous) process. Entropy is a state function described by the equation: where k is a proportionality constant equal t ...
On violations of Le Chatelier`s principle for a temperature change in
... systems, it is not only the average behaviour that is of interest. In this situation the probability of observing a reaction proceed in the opposite direction to that found on average (or in the thermodynamic limit) can be significant. Our derivation will allow us to extend the Principle to describe ...
... systems, it is not only the average behaviour that is of interest. In this situation the probability of observing a reaction proceed in the opposite direction to that found on average (or in the thermodynamic limit) can be significant. Our derivation will allow us to extend the Principle to describe ...
Thermo fundamentals
... Reversible / Irreversible Process Why REVERSIBLE Process ? 1. Easy to analyse, as System passes through series of Equilibriums. 2. Serve as Idealised Model for actual Processes to be compared for analysis. 3. Viewed as Theoretical Limit for corresponding irreversible one. Reversible Process leads to ...
... Reversible / Irreversible Process Why REVERSIBLE Process ? 1. Easy to analyse, as System passes through series of Equilibriums. 2. Serve as Idealised Model for actual Processes to be compared for analysis. 3. Viewed as Theoretical Limit for corresponding irreversible one. Reversible Process leads to ...
Solutions Exercises Lecture 4
... process is adiabatic and isobaric. Note that the process is only adiabatic for the complete system (subsystem A and B) but not for the subsystems A and B. For an isobaric process commonly first the change of the enthalpy is computed and not the change of the internal energy because the equations des ...
... process is adiabatic and isobaric. Note that the process is only adiabatic for the complete system (subsystem A and B) but not for the subsystems A and B. For an isobaric process commonly first the change of the enthalpy is computed and not the change of the internal energy because the equations des ...
heat and temperature
... can measure the latter, we could ask ourselves how bodies are heated. Is the increase in temperature proportional to the energy which we supply in the form of heat? In the following visual we deal with these questions. We have a substance which is heated by a heater the power of which, in W, we can ...
... can measure the latter, we could ask ourselves how bodies are heated. Is the increase in temperature proportional to the energy which we supply in the form of heat? In the following visual we deal with these questions. We have a substance which is heated by a heater the power of which, in W, we can ...