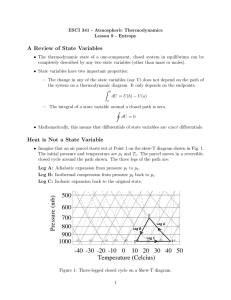
File
... Everest, water boils at 69o. Absolute zero (-273o) is the coldest possible temperature and is used by scientists. The Kelvin scale was developed by William Thomson - a.k.a. Lord Kelvin - and the markings on the scale are not called degrees, but are simply called Kelvins. (0o Celsius is equal to - 27 ...
... Everest, water boils at 69o. Absolute zero (-273o) is the coldest possible temperature and is used by scientists. The Kelvin scale was developed by William Thomson - a.k.a. Lord Kelvin - and the markings on the scale are not called degrees, but are simply called Kelvins. (0o Celsius is equal to - 27 ...
Chapter 6 NOTES!!!!! - Clinton Public Schools
... • Heat moves faster by conduction in solids and liquids than in gases. • In gases, particles are farther apart, so collisions with other particles occur less frequently than they do in solids or liquids. ...
... • Heat moves faster by conduction in solids and liquids than in gases. • In gases, particles are farther apart, so collisions with other particles occur less frequently than they do in solids or liquids. ...
Heat Capacity - Uplift North Hills Prep
... • The interior of roasted meat can never reach temperatures higher than the boiling point of water until all the water is cooked out of it, at which point it would resemble shoe leather. The outside is quickly dried out, however, and can reach the temperature of the surrounding cooking medium. • Coc ...
... • The interior of roasted meat can never reach temperatures higher than the boiling point of water until all the water is cooked out of it, at which point it would resemble shoe leather. The outside is quickly dried out, however, and can reach the temperature of the surrounding cooking medium. • Coc ...
Work Done On or By a Gas
... Section 1 Relationships Between Heat and Work Section 2 The First Law of Thermodynamics Section 3 The Second Law of Thermodynamics ...
... Section 1 Relationships Between Heat and Work Section 2 The First Law of Thermodynamics Section 3 The Second Law of Thermodynamics ...
temperature 2015 10 13
... a family of isolated systems • The fire, the weights and the valve make the water an open system. • Insulate the wall, jam the piston, and shut the valve. Make the water an isolated system. • A system isolated for a long time flips to every quantum state with equal probability. • Entropy S = log (nu ...
... a family of isolated systems • The fire, the weights and the valve make the water an open system. • Insulate the wall, jam the piston, and shut the valve. Make the water an isolated system. • A system isolated for a long time flips to every quantum state with equal probability. • Entropy S = log (nu ...
2. Local equilibrium thermodynamics.
... occurring or can be triggered; within the system, every microscopic process is balanced by its opposite; this is called the principle of detailed balance. A central aim in equilibrium thermodynamics is: given a system in a well-defined initial state, subject to specified constraints, to calculate wh ...
... occurring or can be triggered; within the system, every microscopic process is balanced by its opposite; this is called the principle of detailed balance. A central aim in equilibrium thermodynamics is: given a system in a well-defined initial state, subject to specified constraints, to calculate wh ...
an energy flow analysis between mechanical systems based on the
... kinetic energy). The space r defined by the variables x,,+c, ...
... kinetic energy). The space r defined by the variables x,,+c, ...
Basic Thermodynamics Prof. S. K. Som Department of Mechanical
... amount of work is given by pdv, that means delta W equals that’s why this work will be refer henceforth as pdv work, so if we consider the closed system only performs this type of work that is pdv work, then we can write for that occasion Q1-2 is u2 minus u1 plus integration of pdv between the state ...
... amount of work is given by pdv, that means delta W equals that’s why this work will be refer henceforth as pdv work, so if we consider the closed system only performs this type of work that is pdv work, then we can write for that occasion Q1-2 is u2 minus u1 plus integration of pdv between the state ...
Chapter-9-Handouts
... The object of study. Surrounding: The region outside the system. Internal Energy (E): The capacity to do work or to produce heat. Temperature (T): How hot or cold an object is. Heat (q): The energy that is transferred as a result of a temperature difference between a system and its surroundi ...
... The object of study. Surrounding: The region outside the system. Internal Energy (E): The capacity to do work or to produce heat. Temperature (T): How hot or cold an object is. Heat (q): The energy that is transferred as a result of a temperature difference between a system and its surroundi ...
Thermodynamic Units and Properties Summary
... International System of Units (SI) The SI system is made up of two related systems, the meter-kilogramsecond (MKS) system, and the centimeter-gram-second (CGS) system. The MKS and CGS systems each use a decimal-based system in which prefixes are used to denote powers of ten. For example, one kilome ...
... International System of Units (SI) The SI system is made up of two related systems, the meter-kilogramsecond (MKS) system, and the centimeter-gram-second (CGS) system. The MKS and CGS systems each use a decimal-based system in which prefixes are used to denote powers of ten. For example, one kilome ...
Internal Energy Work Heat
... is in an equilibrium state. This further means that at any instant the change could be reversed and the system returned to its original state (along with the surroundings in as much as they are affected by the system). Such a quasi-static process would need to be performed infinitely slowly and is m ...
... is in an equilibrium state. This further means that at any instant the change could be reversed and the system returned to its original state (along with the surroundings in as much as they are affected by the system). Such a quasi-static process would need to be performed infinitely slowly and is m ...
Statistical mechanics
... values of thermodynamic properties of the system. According to the relationship of the system to the rest of the universe, one of three general types of ensembles may apply, in order of increasing complexity: • Microcanonical ensemble: describes a completely isolated system, having constant energy, ...
... values of thermodynamic properties of the system. According to the relationship of the system to the rest of the universe, one of three general types of ensembles may apply, in order of increasing complexity: • Microcanonical ensemble: describes a completely isolated system, having constant energy, ...