3.1 Thermal concepts (PPT)
... • The interior of roasted meat can never reach temperatures higher than the boiling point of water until all the water is cooked out of it, at which point it would resemble shoe leather. The outside is quickly dried out, however, and can reach the temperature of the surrounding cooking medium. • Coc ...
... • The interior of roasted meat can never reach temperatures higher than the boiling point of water until all the water is cooked out of it, at which point it would resemble shoe leather. The outside is quickly dried out, however, and can reach the temperature of the surrounding cooking medium. • Coc ...
The transformation of a main sequence star into a red
... heat flow enters it . However, the temperature decrease has to be paid for. It can be realized only if the gas expands simultaneously by a sufficient amount. Thermodynamically speaking, a negative heat capacity can only be observed on a system which, in addition to the temperature, has one more degr ...
... heat flow enters it . However, the temperature decrease has to be paid for. It can be realized only if the gas expands simultaneously by a sufficient amount. Thermodynamically speaking, a negative heat capacity can only be observed on a system which, in addition to the temperature, has one more degr ...
This is a heat engine
... 20% and produces an average of 23 kJ of mechanical work per second during operation. Remember: QH = W/e . (a) How much heat input is required, and QH = W/e = 23 kJ/0.20 = 115 kJ (b) How much heat is discharged as waste heat from this engine, per second? QL = (1-e) QH = (0.8) 115 kJ = 92 kJ Copyright ...
... 20% and produces an average of 23 kJ of mechanical work per second during operation. Remember: QH = W/e . (a) How much heat input is required, and QH = W/e = 23 kJ/0.20 = 115 kJ (b) How much heat is discharged as waste heat from this engine, per second? QL = (1-e) QH = (0.8) 115 kJ = 92 kJ Copyright ...
Chapter 4: Energy Analysis of Closed Systems
... One kilogram of water is contained in a piston-cylinder device at 100 C. The piston rests on lower stops such that the volume occupied by the water is 0.835 m3. The cylinder is fitted with an upper set of stops. When the piston rests against the upper stops, the volume enclosed by the piston-cylind ...
... One kilogram of water is contained in a piston-cylinder device at 100 C. The piston rests on lower stops such that the volume occupied by the water is 0.835 m3. The cylinder is fitted with an upper set of stops. When the piston rests against the upper stops, the volume enclosed by the piston-cylind ...
Chapter 2 Classical Thermodynamics: The Second Law 2.1 Heat
... the Otto cycle of Chap. 2.1) are complicated by the fact that they generally need an infinite series of heat reservoirs for the quasistatic processes (e.g., heating at constant volume in the Otto cycle). By contrast, a Carnot engine, by definition is a reversible engine operating between only two he ...
... the Otto cycle of Chap. 2.1) are complicated by the fact that they generally need an infinite series of heat reservoirs for the quasistatic processes (e.g., heating at constant volume in the Otto cycle). By contrast, a Carnot engine, by definition is a reversible engine operating between only two he ...
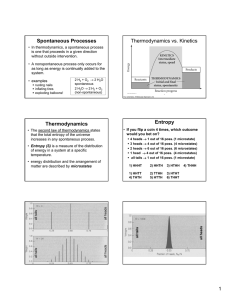
Thermochemistry, thermodynamics Thermochemistry
... The entropy of a substance at any condition is its absolute entropy, also called standard molar entropy. The reference state for absolute entropy is specified by the third law of thermodynamics. It is different from the reference state for ∆Hf0 The standard entropy change, ∆Sr0 , of a reaction can b ...
... The entropy of a substance at any condition is its absolute entropy, also called standard molar entropy. The reference state for absolute entropy is specified by the third law of thermodynamics. It is different from the reference state for ∆Hf0 The standard entropy change, ∆Sr0 , of a reaction can b ...
HEAT - Weebly
... The "zeroth law" states that if two systems are in thermal equilibrium with a third system, then they must be in thermal equilibrium with each other. This law states that if object A is in thermal equilibrium with object B, and object B is in thermal equilibrium with object C, then object C is also ...
... The "zeroth law" states that if two systems are in thermal equilibrium with a third system, then they must be in thermal equilibrium with each other. This law states that if object A is in thermal equilibrium with object B, and object B is in thermal equilibrium with object C, then object C is also ...
Energy
... • In thermodynamics we are also interested in how far a particular reaction goes, and the yield of the reaction as well as factors that will affect these. • There are various forms of energy; light, sound, electrical, heat, nuclear and chemical energy. • Others are mechanical, potential and kinetic ...
... • In thermodynamics we are also interested in how far a particular reaction goes, and the yield of the reaction as well as factors that will affect these. • There are various forms of energy; light, sound, electrical, heat, nuclear and chemical energy. • Others are mechanical, potential and kinetic ...
Consequences of the relation between temperature, heat, and
... a zero point and no possibility of a lower temperature- without much discussion of where it comes from. What defines T = 0 K? o The zero point of the absolute temperature scale was originally derived by identifying the temperature at which heat would be converted into work with 100% efficiency. ...
... a zero point and no possibility of a lower temperature- without much discussion of where it comes from. What defines T = 0 K? o The zero point of the absolute temperature scale was originally derived by identifying the temperature at which heat would be converted into work with 100% efficiency. ...
Fundamental Concepts, Definitions and Zeroth
... 3. Boilers, turbines, heat exchangers. Fluid flow through them and heat or work is taken out or supplied to them. Most of the engineering machines and equipment are open systems. (2) Closed System The system, which can exchange energy with their surrounding but not the mass. The quantity of matter t ...
... 3. Boilers, turbines, heat exchangers. Fluid flow through them and heat or work is taken out or supplied to them. Most of the engineering machines and equipment are open systems. (2) Closed System The system, which can exchange energy with their surrounding but not the mass. The quantity of matter t ...
Chapter 12: Engineering Thermodynamics
... A process is said to be reversible if it is possible for its effects to be eradicated in the sense that there is some way by which both the system and its surroundings can be exactly restored to their respective initial states. A process is irreversible if both the system and surroundings cannot be ...
... A process is said to be reversible if it is possible for its effects to be eradicated in the sense that there is some way by which both the system and its surroundings can be exactly restored to their respective initial states. A process is irreversible if both the system and surroundings cannot be ...
THERMODYNAMICS LECTURE NOTES
... process as shown in figure 1.4. To describe a process completely, one should specify the initial and final states of the process, as well as the path it follows, and the interactions with the surroundings. A process is path followed by a system in reaching a given final state of equilibrium state st ...
... process as shown in figure 1.4. To describe a process completely, one should specify the initial and final states of the process, as well as the path it follows, and the interactions with the surroundings. A process is path followed by a system in reaching a given final state of equilibrium state st ...