documentstyle[12pt]{article}
... The study of thermodynamics is concerned with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an energy interaction, e ...
... The study of thermodynamics is concerned with the ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. One of the most fundamental laws of nature is the conservation of energy principle. It simply states that during an energy interaction, e ...
introduction
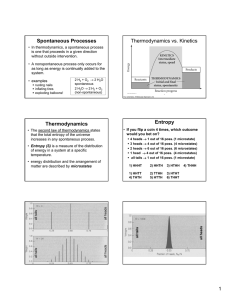
... to another. The second law of thermodynamics is concerned with the reversibility and direction of natural events; it states that the entropy of the universe tends to increase. Therefore, all spontaneous events are accompanied by an increase in the entropy of the universe. Entropy (S) is a measure of ...
... to another. The second law of thermodynamics is concerned with the reversibility and direction of natural events; it states that the entropy of the universe tends to increase. Therefore, all spontaneous events are accompanied by an increase in the entropy of the universe. Entropy (S) is a measure of ...
WRL1834.tmp - Symposium on Chemical Physics
... both sides. As one subsystem does PV work on the other it follows that both the volume and the energy of the subsystems change. If the moveable wall would be kept adiabatic, there is no unique solution for the temperatures of the subsystems. In every text book therefore (but for illuminating discuss ...
... both sides. As one subsystem does PV work on the other it follows that both the volume and the energy of the subsystems change. If the moveable wall would be kept adiabatic, there is no unique solution for the temperatures of the subsystems. In every text book therefore (but for illuminating discuss ...
Energy, work and Power
... Work done/time = ( Force x distance )/ time Work done /time = power and distance/ time = velocity ...
... Work done/time = ( Force x distance )/ time Work done /time = power and distance/ time = velocity ...
the third law of thermodynamics and the low temperature
... This additional mode is associated with the conservation of mass (11). Assuming for simplicity only one kind of atoms there are 8 hydrodynamic variables associated with the Hamiltonian in Eq. ( 8 ) : The momentum density, the energy density, and the mass density. In addition we have three broken sym ...
... This additional mode is associated with the conservation of mass (11). Assuming for simplicity only one kind of atoms there are 8 hydrodynamic variables associated with the Hamiltonian in Eq. ( 8 ) : The momentum density, the energy density, and the mass density. In addition we have three broken sym ...
15-1 Note 15 Properties of Bulk Matter
... Between 0 ˚C and 100 ˚C water is a liquid, and above 100 ˚C it is a gas (in the form of steam or water vapor). The solid, liquid and gaseous states of a substance are called phases. When a substance melts (or freezes) and boils (or condenses) it is said to undergo a phase change. We shall discuss ph ...
... Between 0 ˚C and 100 ˚C water is a liquid, and above 100 ˚C it is a gas (in the form of steam or water vapor). The solid, liquid and gaseous states of a substance are called phases. When a substance melts (or freezes) and boils (or condenses) it is said to undergo a phase change. We shall discuss ph ...
![documentstyle[12pt]{article}](http://s1.studyres.com/store/data/010234315_1-392ad57a1bf5b2aaeca94206588a5307-300x300.png)