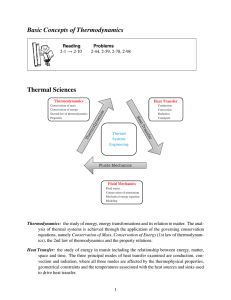
A System and Its Surroundings
... There are many different types of energy, but the two that will be discussed in this section are potential energy and kinetic energy. Potential energy is "stored energy," energy that contains the potential to do work when released. Any object that is stationary contains potential energy. For example ...
... There are many different types of energy, but the two that will be discussed in this section are potential energy and kinetic energy. Potential energy is "stored energy," energy that contains the potential to do work when released. Any object that is stationary contains potential energy. For example ...
Chapter Entropy Statistics
... The thermodynamic probability W is a function of no. of particles (n) (which is function of µ amount of substance, the total no. of available phase space cells (which in turn depends upon the volume) and the energy u of the system. ...
... The thermodynamic probability W is a function of no. of particles (n) (which is function of µ amount of substance, the total no. of available phase space cells (which in turn depends upon the volume) and the energy u of the system. ...
CHAPTER 9: Statistical Physics
... Ludwig Boltzmann, who spent much of his life studying statistical mechanics, died in 1906 by his own hand. Paul Ehrenfest, carrying on his work, died similarly in 1933. Now it is our turn to study statistical mechanics. Perhaps it will be wise to approach the subject cautiously. - David L. Goldstein ...
... Ludwig Boltzmann, who spent much of his life studying statistical mechanics, died in 1906 by his own hand. Paul Ehrenfest, carrying on his work, died similarly in 1933. Now it is our turn to study statistical mechanics. Perhaps it will be wise to approach the subject cautiously. - David L. Goldstein ...
Biochemistry 304 2014 Student Edition Thermodynamics Lecture
... If the only work done is by volume change (pdV) Then ...
... If the only work done is by volume change (pdV) Then ...
1. What are the two most abundant permanent gasses, and roughly
... 27.Do greenhouse gases warm the earth by absorbing sunlight? 28.What are the two most important greenhouse gases on Earth? 29.Why do we expect increasing concentrations of carbon dioxide should lead to "global warming"? 30.Explain why clouds tend to keep the surface temperature warmer at night but c ...
... 27.Do greenhouse gases warm the earth by absorbing sunlight? 28.What are the two most important greenhouse gases on Earth? 29.Why do we expect increasing concentrations of carbon dioxide should lead to "global warming"? 30.Explain why clouds tend to keep the surface temperature warmer at night but c ...
Experiment 1 - 8. Form of Energy
... although they passed different courses. It corresponds to the transfer of water in a dam, in which the amount of water is changed not only by the in-and-out process on the gate but also by the rain or evaporation. But the water from different sources can't be distinguished. ...
... although they passed different courses. It corresponds to the transfer of water in a dam, in which the amount of water is changed not only by the in-and-out process on the gate but also by the rain or evaporation. But the water from different sources can't be distinguished. ...
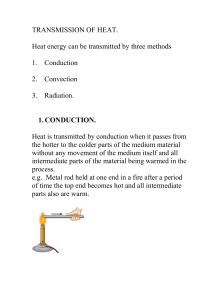
Lecture 5: Heat transmission
... P = Heat loss rating = Rate of energy flow through the element = Power measured in Watts. A = Area of the material measured in m2. T = temperature difference measured in oC or Kelvin ...
... P = Heat loss rating = Rate of energy flow through the element = Power measured in Watts. A = Area of the material measured in m2. T = temperature difference measured in oC or Kelvin ...