The Atom and the Ion
... atom (give) are changed into a positive ion with equivalent number of positive charges to the given electrons. ...
... atom (give) are changed into a positive ion with equivalent number of positive charges to the given electrons. ...
energy levels
... Assumption 3: Radiation is emitted by the atom when the electron makes a transition from an initial state to a lower-energy orbit. The frequency of the emitted radiation is given by Ei – Ef = hƒ, which is independent of frequency of the electron’s orbital motion. Assumption 4: The allowed orbits ar ...
... Assumption 3: Radiation is emitted by the atom when the electron makes a transition from an initial state to a lower-energy orbit. The frequency of the emitted radiation is given by Ei – Ef = hƒ, which is independent of frequency of the electron’s orbital motion. Assumption 4: The allowed orbits ar ...
Atomic Structure: SOL Review #1 Name: Historical Developments 1
... all things made of tiny particles solid sphere model; Atomic Theory: all elements made of atoms, atoms of an element are identical cathode ray tube; discovered electron; plum pudding model oil drop; discovered mass and charge of electron gold foil; discovered nucleus; the atom is mostly empty space ...
... all things made of tiny particles solid sphere model; Atomic Theory: all elements made of atoms, atoms of an element are identical cathode ray tube; discovered electron; plum pudding model oil drop; discovered mass and charge of electron gold foil; discovered nucleus; the atom is mostly empty space ...
Midterm Review
... will identify areas that you need to concentrate on for the final. Go through your notes and worksheets to help you answer the rest of the questions. Chapters 2 and 3 – MATTER ...
... will identify areas that you need to concentrate on for the final. Go through your notes and worksheets to help you answer the rest of the questions. Chapters 2 and 3 – MATTER ...
ATOMS, MOLECULES and IONS
... In a simple ion, one nucleus is present, but the species carries a charge because the number of electrons does not equal the +ve charge on the nucleus. This means that the atom has either lost or gained one or more electrons........ A gain of electrons results in a negatively charged ion; known as a ...
... In a simple ion, one nucleus is present, but the species carries a charge because the number of electrons does not equal the +ve charge on the nucleus. This means that the atom has either lost or gained one or more electrons........ A gain of electrons results in a negatively charged ion; known as a ...
Chapter 9 - "Atomic Structure"
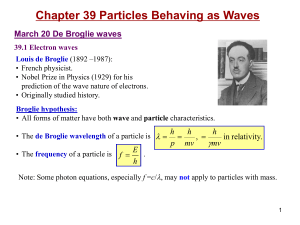
... – In 1900 Max Plank introduced the idea that matter emits and absorbs energy in discrete units called quanta. – In 1905 Albert Einstein extended the quantum concept to include light and that light consist of discrete units called photons. – The energy of a photon is directly proportional to the freq ...
... – In 1900 Max Plank introduced the idea that matter emits and absorbs energy in discrete units called quanta. – In 1905 Albert Einstein extended the quantum concept to include light and that light consist of discrete units called photons. – The energy of a photon is directly proportional to the freq ...
Separation of internal and center-of
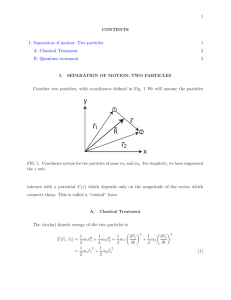
... Thus, since the potential depends only on |~r|, the Hamiltonian separates into a term describing the kinetic energy of the center-of-mass, moving through space with mass M, and the kinetic energy and potential energy of the relative motion of particle 2 with respect to particle 1, with the kinetic e ...
... Thus, since the potential depends only on |~r|, the Hamiltonian separates into a term describing the kinetic energy of the center-of-mass, moving through space with mass M, and the kinetic energy and potential energy of the relative motion of particle 2 with respect to particle 1, with the kinetic e ...
The Atom
... What are the similarities and differences of the atomic models of Democritus, Aristotle, and Dalton? How was Dalton’s theory used to explain the conservation of mass? What is an atom? How can the subatomic particles be distinguished in terms of relative charge and mass? What is an isotope? Giv ...
... What are the similarities and differences of the atomic models of Democritus, Aristotle, and Dalton? How was Dalton’s theory used to explain the conservation of mass? What is an atom? How can the subatomic particles be distinguished in terms of relative charge and mass? What is an isotope? Giv ...
Physics 12 Assignmen.. - hrsbstaff.ednet.ns.ca
... 2. In Rutherford’s planetary model of the atom, what keeps the electrons from flying off into space? In Rutherford’s planetary model of the atom, the Coulomb (or electrostatic) force keeps the electrons from flying off into space. Since the protons in the center are positively charged, the negativel ...
... 2. In Rutherford’s planetary model of the atom, what keeps the electrons from flying off into space? In Rutherford’s planetary model of the atom, the Coulomb (or electrostatic) force keeps the electrons from flying off into space. Since the protons in the center are positively charged, the negativel ...
Ch. 5 Notes: Electrons in Atoms Big Idea: The Atoms of each
... a. Quantum- the minimum amount of energy that can be gained or lost by an atom. 3. Energy of a Quantum a. E quantum = hv ...
... a. Quantum- the minimum amount of energy that can be gained or lost by an atom. 3. Energy of a Quantum a. E quantum = hv ...
Electron Orbits
... theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin ...
... theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin ...
Emission Spectroscopy Lab
... The normal electron configuration of an atom or ion(s) of an element are known as the “ground state”. In the most stable energy state, all electrons are in the lowest energy state possible. (According to the AUFBAU Principle, electrons will occupy the lowest energy state possible.) When energy is ad ...
... The normal electron configuration of an atom or ion(s) of an element are known as the “ground state”. In the most stable energy state, all electrons are in the lowest energy state possible. (According to the AUFBAU Principle, electrons will occupy the lowest energy state possible.) When energy is ad ...
Activity Name: Polyatomic Ion Bingo
... Background Information: An ion is an atom or group of atoms that has a charge because of the loss or gain of electrons. A polyatomic ion is an ion that consists of at least two different elements. Polyatomic ions are common ingredients in many foods and household products. Phosphate, nitrate, sulfat ...
... Background Information: An ion is an atom or group of atoms that has a charge because of the loss or gain of electrons. A polyatomic ion is an ion that consists of at least two different elements. Polyatomic ions are common ingredients in many foods and household products. Phosphate, nitrate, sulfat ...
Name: Period
... 1. Describe the quantum mechanical model of an atom? 2. Which scientist developed the quantum mechanical model of an atom? 3. What are the shapes of an s and p orbitals? 4. What is a principal energy level, sublevel and atomic orbital? 5. What is the maximum number in each s, p, d and f orbitals? 6. ...
... 1. Describe the quantum mechanical model of an atom? 2. Which scientist developed the quantum mechanical model of an atom? 3. What are the shapes of an s and p orbitals? 4. What is a principal energy level, sublevel and atomic orbital? 5. What is the maximum number in each s, p, d and f orbitals? 6. ...
There are a total of n subshells, each specified by an
... Chemical properties of an atom are determined by the least tightly bound electrons. Factors: •Occupancy of subshell •Energy separation between the subshell and the next higher subshell. ...
... Chemical properties of an atom are determined by the least tightly bound electrons. Factors: •Occupancy of subshell •Energy separation between the subshell and the next higher subshell. ...
CM1111* Question 1 (40 marks) Multiple Choice Questions, 5 marks
... A. In order of increasing ionisation energy, Mg
... A. In order of increasing ionisation energy, Mg
Midterm Exam 2
... Exam 2 –answer key Section 1- Concepts and Definitions (50% 5 points each) 1) Give two examples of a hydrogenic atom other than hydrogen (H): ...
... Exam 2 –answer key Section 1- Concepts and Definitions (50% 5 points each) 1) Give two examples of a hydrogenic atom other than hydrogen (H): ...
The “classically forbidden regions” are where … a. a particle`s total
... (takes more energy) • You can instead change their internal quantum numbers (if they have them). • Electrons do have 1 internal number that can be +1/2 or -1/2 so 2 of them can get into a state. ...
... (takes more energy) • You can instead change their internal quantum numbers (if they have them). • Electrons do have 1 internal number that can be +1/2 or -1/2 so 2 of them can get into a state. ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.