Chapter 2
... 1. # protons = atomic number (unique for each element) 2. # protons + # neutrons = atomic mass ...
... 1. # protons = atomic number (unique for each element) 2. # protons + # neutrons = atomic mass ...
physics - Keith E. Holbert
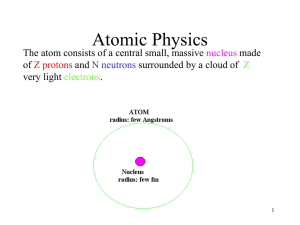
... Z: atomic number (the number of protons), N: neutron number (the number of neutrons), and A=N+Z: atomic mass number (the number of nucleons). Definitions and Distinctions • atomic mass number (A) [integer number] vs. atomic weight or atomic mass (M) [real value]: M≅A • isotope: nuclides with equal n ...
... Z: atomic number (the number of protons), N: neutron number (the number of neutrons), and A=N+Z: atomic mass number (the number of nucleons). Definitions and Distinctions • atomic mass number (A) [integer number] vs. atomic weight or atomic mass (M) [real value]: M≅A • isotope: nuclides with equal n ...
Semester Exam Practice Questions
... 48. The formula mass of magnesium chloride, MgCl2, is __________. a. 59.8 amu c. 95.2 amu b. 76.4 amu d. 125.8 amu 49. If one molecule of NH3 has a mass of 17.0 g/mol, what is the mass of 6.02 x 1023 molecules of NH3? a. 2.82 g c. 102 g b. 17.0 g d. 2.82 x 10-25 g 50. Which of the following statemen ...
... 48. The formula mass of magnesium chloride, MgCl2, is __________. a. 59.8 amu c. 95.2 amu b. 76.4 amu d. 125.8 amu 49. If one molecule of NH3 has a mass of 17.0 g/mol, what is the mass of 6.02 x 1023 molecules of NH3? a. 2.82 g c. 102 g b. 17.0 g d. 2.82 x 10-25 g 50. Which of the following statemen ...
Hydrogen Atom Energy Levels
... • The IE for a hydrogen atom in the ground state = ______________ J. This means if you put that amount of energy into a hydrogen atom in its ground state, the electron is no longer bound to the nucleus. • The IE for a hydrogen atom in the n = 2 (first excited state) is ______________ J. • The IE ...
... • The IE for a hydrogen atom in the ground state = ______________ J. This means if you put that amount of energy into a hydrogen atom in its ground state, the electron is no longer bound to the nucleus. • The IE for a hydrogen atom in the n = 2 (first excited state) is ______________ J. • The IE ...
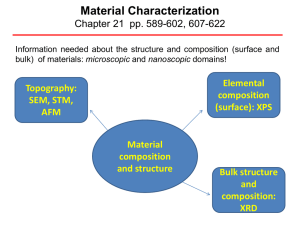
Material Characterization
... X-ray Photoelectron Spectroscopy (XPS) XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its "as received" state, or after some treatment XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot detect hyd ...
... X-ray Photoelectron Spectroscopy (XPS) XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its "as received" state, or after some treatment XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot detect hyd ...
A. Atomic and Nuclear Structure
... atom. A charged atom is known as an ion. Ions readily form chemical bonds with other ions of opposing charge. Although electrons exist in a cloud around the nucleus, it is useful to describe this arrangement as a series of energy levels, called shells. Within each shell are subgroups of electrons, c ...
... atom. A charged atom is known as an ion. Ions readily form chemical bonds with other ions of opposing charge. Although electrons exist in a cloud around the nucleus, it is useful to describe this arrangement as a series of energy levels, called shells. Within each shell are subgroups of electrons, c ...
8th Grade: First Semester Final Review
... 40. a starting substance in a chemical reaction 42. a number placed in front of an element symbol or chemical formula in an equation 41. a substance produced in a chemical reaction 43. a process in which atoms of one or more substances rearrange to form one or more new substances Short Answer 1. Ide ...
... 40. a starting substance in a chemical reaction 42. a number placed in front of an element symbol or chemical formula in an equation 41. a substance produced in a chemical reaction 43. a process in which atoms of one or more substances rearrange to form one or more new substances Short Answer 1. Ide ...
Notes on Atomic Structure atoms
... same proportions (by mass and by number) of its elements This means a given compound always has the same composition, regardless of where it came from. ...
... same proportions (by mass and by number) of its elements This means a given compound always has the same composition, regardless of where it came from. ...
FXM Rev 1 Key - Grande Cache Community High School
... Rutherford’s model This model formed as a result of the gold foil experiment. It involves a positively charged nucleus with electrons in orbit. It is sometimes called the Planetary Atomic Model. hydrocarbons These are organic compounds that contain both carbon and hydrogen. Methane (CH4) is an exam ...
... Rutherford’s model This model formed as a result of the gold foil experiment. It involves a positively charged nucleus with electrons in orbit. It is sometimes called the Planetary Atomic Model. hydrocarbons These are organic compounds that contain both carbon and hydrogen. Methane (CH4) is an exam ...
CHAPTER 3 Atoms: The Building Blocks of Matter
... – All matter is composed of extremely small particles called atoms – Atoms of a given element are identical in size, mass, and other properties – Atoms cannot be subdivided, created, or destroyed – Atoms of different elements combine in simple whole number ratios to form compounds – In chemical reac ...
... – All matter is composed of extremely small particles called atoms – Atoms of a given element are identical in size, mass, and other properties – Atoms cannot be subdivided, created, or destroyed – Atoms of different elements combine in simple whole number ratios to form compounds – In chemical reac ...
Notes - Ms. Dawkins
... An atom is made up of 3 _________________ particles: 1. Protons—have a __________________ (+) charge 2. Neutrons—have _________________ (o) charge (think: neutral) 3. Electrons—have a _________________ (-) charge Particles with the same type of charge __________________ each other—they push away fro ...
... An atom is made up of 3 _________________ particles: 1. Protons—have a __________________ (+) charge 2. Neutrons—have _________________ (o) charge (think: neutral) 3. Electrons—have a _________________ (-) charge Particles with the same type of charge __________________ each other—they push away fro ...
Chapter Outline • Review of Atomic Structure Electrons, protons
... The number of atoms in a mole is called the Avogadro number, Nav = 6.023 × 10 23. 1 amu/atom = 1 gram/mol Example: Atomic weight of iron = 55.85 amu/atom = 55.85 g/mol ...
... The number of atoms in a mole is called the Avogadro number, Nav = 6.023 × 10 23. 1 amu/atom = 1 gram/mol Example: Atomic weight of iron = 55.85 amu/atom = 55.85 g/mol ...
Exam 3
... 19. The number of Fermions (such as electrons) that can be in a particular quantum state: a. one b. two A fermion is a particle with half-integer spin c. any number (e.g ½, 3/2, 5/2, etc). Only one fermion can be d. any number except zero in a particular quantum state. e. depends on the spin of the ...
... 19. The number of Fermions (such as electrons) that can be in a particular quantum state: a. one b. two A fermion is a particle with half-integer spin c. any number (e.g ½, 3/2, 5/2, etc). Only one fermion can be d. any number except zero in a particular quantum state. e. depends on the spin of the ...
PracticeQuestions
... Within a group of elements, as the atomic number increases, the atomic radius ____. A. generally increases C. decreases regularly B. remains generally constant D. decreases, but not regularly For each electron removed from an atom, the ionization energy ____ A. increases C. remains the same B. decre ...
... Within a group of elements, as the atomic number increases, the atomic radius ____. A. generally increases C. decreases regularly B. remains generally constant D. decreases, but not regularly For each electron removed from an atom, the ionization energy ____ A. increases C. remains the same B. decre ...
Atomic Structure
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
Chemistry I Review - BarbaraElam-Rice
... 30) An atom has 2 electrons in its valence shell. To what group does the atom belong? Will the atom for an anion or a cation? What will be the oxidation number of the ion? 31) An intermolecular force that holds ionic compounds together is called electrostatic attraction. 32) Describe the 3 intermole ...
... 30) An atom has 2 electrons in its valence shell. To what group does the atom belong? Will the atom for an anion or a cation? What will be the oxidation number of the ion? 31) An intermolecular force that holds ionic compounds together is called electrostatic attraction. 32) Describe the 3 intermole ...
chapter02_part1_lecture - bloodhounds Incorporated
... • The first shell (closest to the nucleus) can contain two electrons • Each additional shell can contain eight electrons • Each lower shell is filled with electrons ...
... • The first shell (closest to the nucleus) can contain two electrons • Each additional shell can contain eight electrons • Each lower shell is filled with electrons ...
On the Ionization Energy of the Outer Electrons of Atoms and Their
... n , may mean, for example, that electronic shells (layers) are “spatially structured”: the electrons experience something like random “migration” between nodes of a certain spatial lattice (with the number of vertexes 2n 2 ), inscribed into a sphere of radius rn . For example, for n = 2 the “structu ...
... n , may mean, for example, that electronic shells (layers) are “spatially structured”: the electrons experience something like random “migration” between nodes of a certain spatial lattice (with the number of vertexes 2n 2 ), inscribed into a sphere of radius rn . For example, for n = 2 the “structu ...
Collision Theory
... Theories of Chemical Kinetics: Collision Theory • Before atoms/molecules/ions can react, they must first collide • An effective collision between two species puts enough energy to break key bonds • The activation energy (Ea) is the minimum energy that must be supplied by collisions to trigger a rea ...
... Theories of Chemical Kinetics: Collision Theory • Before atoms/molecules/ions can react, they must first collide • An effective collision between two species puts enough energy to break key bonds • The activation energy (Ea) is the minimum energy that must be supplied by collisions to trigger a rea ...
Miss Pang`s 2012 Review
... 19. In 1803, John Dalton, in an attempt to explain the findings of his work on the solubility of gases as well as the laws of Lav oiser and Proust, wrote the first atomic theory based on experimental fact. Which of the following statements does not correspond with Dalton’s theory? ...
... 19. In 1803, John Dalton, in an attempt to explain the findings of his work on the solubility of gases as well as the laws of Lav oiser and Proust, wrote the first atomic theory based on experimental fact. Which of the following statements does not correspond with Dalton’s theory? ...
Why The Sky Is Blue
... much more complex than described here, of course; there are subtle dependences of color and intensity as a function of angle from the sun, polarization of the light, dust is typically not small compared to the wavelengths of light, and so Rayleigh scattering is not occurring, etc. Rayleigh scatterin ...
... much more complex than described here, of course; there are subtle dependences of color and intensity as a function of angle from the sun, polarization of the light, dust is typically not small compared to the wavelengths of light, and so Rayleigh scattering is not occurring, etc. Rayleigh scatterin ...
Exam #: Printed Name: Signature: PHYSICS DEPARTMENT
... The examination papers are numbered in the upper right-hand corner of each page. Print and then sign your name in the spaces provided on this page. For identification purposes, be sure to submit this page together with your answers when the exam is finished. Be sure to place both the exam number and ...
... The examination papers are numbered in the upper right-hand corner of each page. Print and then sign your name in the spaces provided on this page. For identification purposes, be sure to submit this page together with your answers when the exam is finished. Be sure to place both the exam number and ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.