ACA__Beat_sheet_bonding_2016
... What are some properties of metals? What are some properties of nonmetals? In what block (s, p, d, f) are the Lanthanides and Actinides? ...
... What are some properties of metals? What are some properties of nonmetals? In what block (s, p, d, f) are the Lanthanides and Actinides? ...
Bohr Atom
... classical mechanics (i.e. the Larmor formula, power radiated by a charged particle as it accelerates.), predict that the electron will release electromagnetic radiation while orbiting a nucleus. Because the electron would lose energy, it would gradually spiral inwards, collapsing into the nucleus. T ...
... classical mechanics (i.e. the Larmor formula, power radiated by a charged particle as it accelerates.), predict that the electron will release electromagnetic radiation while orbiting a nucleus. Because the electron would lose energy, it would gradually spiral inwards, collapsing into the nucleus. T ...
Electrons in Atoms
... But it isn’t like this! The reality is much more complex! The behaviour of electrons in atoms can be derived and described using quantum mechanics (QM). At this stage we are not going to be concerned with the mathematical aspects of QM, but we will study in detail the results and concepts yiel ...
... But it isn’t like this! The reality is much more complex! The behaviour of electrons in atoms can be derived and described using quantum mechanics (QM). At this stage we are not going to be concerned with the mathematical aspects of QM, but we will study in detail the results and concepts yiel ...
3rd Quarter Test
... 16) The percent by mass of oxygen in MgO (gram formula mass = 40) is closest to a) 16 % b) 24 % c) 40 % d) 60 % 17) What is the mass in grams of 2.00 moles of CaCl2? a) 1.00 b) 2.00 c) 111 ...
... 16) The percent by mass of oxygen in MgO (gram formula mass = 40) is closest to a) 16 % b) 24 % c) 40 % d) 60 % 17) What is the mass in grams of 2.00 moles of CaCl2? a) 1.00 b) 2.00 c) 111 ...
AP Quantum physics
... Example 3: In an experiment to determine Planck’s constant, a plot of stopping potential versus frequency is made. The slope of the curve is 4.13 x 10-15 V/Hz. What is Planck’s constant? ...
... Example 3: In an experiment to determine Planck’s constant, a plot of stopping potential versus frequency is made. The slope of the curve is 4.13 x 10-15 V/Hz. What is Planck’s constant? ...
Chapter 13 Spectroscopy NMR, IR, MS, UV-Vis
... against the field), those fields would affect the effective field felt by the hydrogen being measured. In the high energy state they would oppose (reduce) the field and in the low energy state reinforce (increase) the field. Thus a neighboring hydrogen would cause another hydrogen to feel two fields ...
... against the field), those fields would affect the effective field felt by the hydrogen being measured. In the high energy state they would oppose (reduce) the field and in the low energy state reinforce (increase) the field. Thus a neighboring hydrogen would cause another hydrogen to feel two fields ...
Bohr`s atomic model
... of the hydrogen atom, but there was no clear physical reasoning to support his assumption of angular momentum quantization. 9. “New” quantum theory considers the electron to be a wave. It discards the notion of orbits and replaces it with the notion of orbitals. Orbitals are stationary (standing) wa ...
... of the hydrogen atom, but there was no clear physical reasoning to support his assumption of angular momentum quantization. 9. “New” quantum theory considers the electron to be a wave. It discards the notion of orbits and replaces it with the notion of orbitals. Orbitals are stationary (standing) wa ...
Bohr`s Model of the Atom
... nucleus in particular circular orbits with fixed energy, its distance from the nucleus being proportional to its energy. • Under this model an electron could not spiral into the nucleus because it could not lose energy in a continuous manner; instead, it could only make instantaneous "quantum leaps" ...
... nucleus in particular circular orbits with fixed energy, its distance from the nucleus being proportional to its energy. • Under this model an electron could not spiral into the nucleus because it could not lose energy in a continuous manner; instead, it could only make instantaneous "quantum leaps" ...
form revision a
... Check your key area statements. If not green you need to do more work! Knowledge of the structure of the periodic table, groups and periods. All matter is made of atoms. When a substance contains only one kind of atom it is known as an element. Atoms contain protons, neutrons and electrons each with ...
... Check your key area statements. If not green you need to do more work! Knowledge of the structure of the periodic table, groups and periods. All matter is made of atoms. When a substance contains only one kind of atom it is known as an element. Atoms contain protons, neutrons and electrons each with ...
AP Exam Two Retake Qualifying Assignment
... d. both clockwise and counterclockwise What types of atomic orbitals are in the fourth principal energy level? a. s and p only c. s, p, and d only b. p and d only d. s, p, d, and f What is the next atomic orbital in the series 1s, 2s, 2p, 3s, 3p? a. 2d c. 3f b. 3d d. 4s If three electrons are availa ...
... d. both clockwise and counterclockwise What types of atomic orbitals are in the fourth principal energy level? a. s and p only c. s, p, and d only b. p and d only d. s, p, d, and f What is the next atomic orbital in the series 1s, 2s, 2p, 3s, 3p? a. 2d c. 3f b. 3d d. 4s If three electrons are availa ...
Raman_Intensities
... •Arm waving explanation, you can get Raman intensity if the vibration of the atoms causes a change in the polarization of the electron density at the macro scale. Of course every vibration of an atom causes a change in the polarization of the electron density at the atomic scale. •Important case, if ...
... •Arm waving explanation, you can get Raman intensity if the vibration of the atoms causes a change in the polarization of the electron density at the macro scale. Of course every vibration of an atom causes a change in the polarization of the electron density at the atomic scale. •Important case, if ...
Compact Beam Steering
... In the interest of working towards these goals, a single-element, single-wavelength beam steering system was built under the Phase I contract. This breadboard system enabled us to assess system performance in terms of optical transmission, tracking bandwidth, Size, Weight and Power (SWaP) and steeri ...
... In the interest of working towards these goals, a single-element, single-wavelength beam steering system was built under the Phase I contract. This breadboard system enabled us to assess system performance in terms of optical transmission, tracking bandwidth, Size, Weight and Power (SWaP) and steeri ...
Quantum physics
... Knowledge about atomic spectra can be very useful in some situations. By looking at the radiation of a distant star, you can determine what gases are in the star. Or let’s pretend you want to have a light source that produces only specific colors: You can decide what type of gas lamp to use. This i ...
... Knowledge about atomic spectra can be very useful in some situations. By looking at the radiation of a distant star, you can determine what gases are in the star. Or let’s pretend you want to have a light source that produces only specific colors: You can decide what type of gas lamp to use. This i ...
Electrons and Photons
... • Photons of all colors can be emitted. • All colors blend into “white light” ...
... • Photons of all colors can be emitted. • All colors blend into “white light” ...
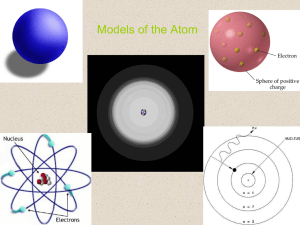
Models of the Atom
... – The quantum mechanical model (electron cloud model): • The modern description of electrons in an atom • Determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. • Based on probability ...
... – The quantum mechanical model (electron cloud model): • The modern description of electrons in an atom • Determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. • Based on probability ...
Energy and Matter
... elements bonded together. (ex: NaCl, H2O) Pure substances are elements & compounds. ...
... elements bonded together. (ex: NaCl, H2O) Pure substances are elements & compounds. ...
Wave Particle Duality Power Point NOTES
... Waves -NOT SYNCHRONIZED - waves cancel out result black/gray bands ...
... Waves -NOT SYNCHRONIZED - waves cancel out result black/gray bands ...
Electron Arrangement
... These shells are filled until all the electrons are placed, giving the electron arrangement of the atom. Eg, Sodium has 11 electrons so has an electron arrangement of 2,8,1. Elements in the same group of the Periodic Table have the same number of electrons in their outer electron shell. Isotopes The ...
... These shells are filled until all the electrons are placed, giving the electron arrangement of the atom. Eg, Sodium has 11 electrons so has an electron arrangement of 2,8,1. Elements in the same group of the Periodic Table have the same number of electrons in their outer electron shell. Isotopes The ...
The Atomic Theory
... The chemical action of an electric current is directly proportional to the quantity of electricity which passes through a solution. The weights of the substances deposited by the same quantity of electricity are proportional to their chemical equivalents. Stoney (1874) made the hypothesis that there ...
... The chemical action of an electric current is directly proportional to the quantity of electricity which passes through a solution. The weights of the substances deposited by the same quantity of electricity are proportional to their chemical equivalents. Stoney (1874) made the hypothesis that there ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.