Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... Law of Constant Composition (Definite Proportions) "In a compound, elements are always present in a definite proportion by weight." Thus, water, a compound is always 11 parts H and 89 parts O by weight (mass) John Dalton’s Atomic Theory (1803-1807) A comprehensive theory (a synthesis) to account for ...
... Law of Constant Composition (Definite Proportions) "In a compound, elements are always present in a definite proportion by weight." Thus, water, a compound is always 11 parts H and 89 parts O by weight (mass) John Dalton’s Atomic Theory (1803-1807) A comprehensive theory (a synthesis) to account for ...
Solutions - Dynamic Science
... The smallest particle of matter. The smallest possible sugar crystal. The smallest particle of water. The energy given off during a chemical reaction. ...
... The smallest particle of matter. The smallest possible sugar crystal. The smallest particle of water. The energy given off during a chemical reaction. ...
Lecture28
... Quantization of angular momentum and de Broglie waves • De Broglie found an interpretation of the Bohr’s angular momentum quantization in terms of his wave theory. An electron orbit would be stable (allowed) only if it contained an integral number of electron wavelengths. ...
... Quantization of angular momentum and de Broglie waves • De Broglie found an interpretation of the Bohr’s angular momentum quantization in terms of his wave theory. An electron orbit would be stable (allowed) only if it contained an integral number of electron wavelengths. ...
CHEMISTRY: MIDTERM EXAM REVIEW SPRING 2013 Multiple
... ____ 15. Which of the following electron transitions for the hydrogen atom would be associated with the greatest energy of emitted light? a. n = 5 to n = 1 c. n = 4 to n = 5 b. n = 5 to n = 4 d. n = 2 to n = 5 ____ 16. In which of the following sets are the charges given correctly for all the ions? ...
... ____ 15. Which of the following electron transitions for the hydrogen atom would be associated with the greatest energy of emitted light? a. n = 5 to n = 1 c. n = 4 to n = 5 b. n = 5 to n = 4 d. n = 2 to n = 5 ____ 16. In which of the following sets are the charges given correctly for all the ions? ...
Matter is anything that occupies space and has mass. Examples
... Density is the mass per unit of volume. It is affected by a change in temperature. Formula: Density = mass D=m Volume V ...
... Density is the mass per unit of volume. It is affected by a change in temperature. Formula: Density = mass D=m Volume V ...
The Wizard Test Maker
... (E) Number of occupied electron shells in the ground state 3. Two isotopes of uranium are U-237 and U-238. Both would be expected to have the same (A) mass (D) number of neutrons (B) number of protons (E) half-life (C) decay mode 4. Whose gold foil experiment concluded that the positive charge of an ...
... (E) Number of occupied electron shells in the ground state 3. Two isotopes of uranium are U-237 and U-238. Both would be expected to have the same (A) mass (D) number of neutrons (B) number of protons (E) half-life (C) decay mode 4. Whose gold foil experiment concluded that the positive charge of an ...
Matter - Moodle
... • Helium is light and non-flammable so it is good for _____________________ element A substance that cannot be separated or broken down into simpler substances by __________________ means More than _______elements occur naturally on Earth ...
... • Helium is light and non-flammable so it is good for _____________________ element A substance that cannot be separated or broken down into simpler substances by __________________ means More than _______elements occur naturally on Earth ...
Assignment 8 - Duke Physics
... The balloon-like shapes represent surfaces of large electron probability density |Ψ|2 where the electron in that orbital has a large probability of being found inside the balloon; we will learn later in this course how to calculate these shapes mathematically when we solve the Schrodinger equation f ...
... The balloon-like shapes represent surfaces of large electron probability density |Ψ|2 where the electron in that orbital has a large probability of being found inside the balloon; we will learn later in this course how to calculate these shapes mathematically when we solve the Schrodinger equation f ...
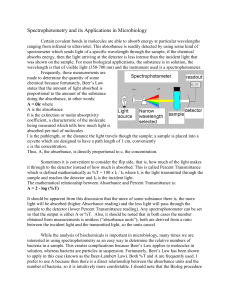
Spectrophotometry and its Applications in Microbiology
... spectrometer which sends light of a specific wavelength through the sample; if the chemical absorbs energy, then the light arriving at the detector is less intense than the incident light that was shown on the sample. For most biological applications, the substance is in solution, the wavelength is ...
... spectrometer which sends light of a specific wavelength through the sample; if the chemical absorbs energy, then the light arriving at the detector is less intense than the incident light that was shown on the sample. For most biological applications, the substance is in solution, the wavelength is ...
Measuring and Calculating
... Because gases have more ranges of motion than liquids and solids, at constant temperature gases tend to have more entropy followed by liquids and solids example: An ice cube is placed on a hot skillet. 1st as it melts the entropy increases, 2nd as the liquid evaporates the entropy increases furt ...
... Because gases have more ranges of motion than liquids and solids, at constant temperature gases tend to have more entropy followed by liquids and solids example: An ice cube is placed on a hot skillet. 1st as it melts the entropy increases, 2nd as the liquid evaporates the entropy increases furt ...
Mass Spectrometry and Organic
... Mass spectrometry “sees” all the isotopomers as distinct ions An ion with all 12C is one mass unit different from an ion with one 13C and the rest 12C Since the isotope distribution in nature is known* for all the elements (13C is 1.1%), the anticipated range and ratios of ions for a given formula c ...
... Mass spectrometry “sees” all the isotopomers as distinct ions An ion with all 12C is one mass unit different from an ion with one 13C and the rest 12C Since the isotope distribution in nature is known* for all the elements (13C is 1.1%), the anticipated range and ratios of ions for a given formula c ...
Chemistry Unit Test Study Guide (2012-2013)
... The left side of a chemical reaction is made up of reactants / products, the right side is reactants / products. f. Energy Transfer- Forms of energy: ___________________ ____________________ A change that gives off energy is called ________________________________ Example _____________________ ...
... The left side of a chemical reaction is made up of reactants / products, the right side is reactants / products. f. Energy Transfer- Forms of energy: ___________________ ____________________ A change that gives off energy is called ________________________________ Example _____________________ ...
Nuclear Chemistry PowerPoint
... parts, releasing a large amount of energy in the process. Most commonly this is done by "firing" a neutron at the nucleus of an atom. The energy of the neutron "bullet" causes the target element to split into two (or more) elements that are lighter than the parent atom. • During the fission of U235, ...
... parts, releasing a large amount of energy in the process. Most commonly this is done by "firing" a neutron at the nucleus of an atom. The energy of the neutron "bullet" causes the target element to split into two (or more) elements that are lighter than the parent atom. • During the fission of U235, ...
3.7 Dielectrics and Optics 3.7.1 Basics
... The incident beam also has a certain amplitude of the electric field (and of the magnetic field, of course) which we call E0. The intensity Ii of the light that the incident beams embodies, i.e. the energy flow, is proportional to E02 - never mix up the two! The reflected beam follows one of the bas ...
... The incident beam also has a certain amplitude of the electric field (and of the magnetic field, of course) which we call E0. The intensity Ii of the light that the incident beams embodies, i.e. the energy flow, is proportional to E02 - never mix up the two! The reflected beam follows one of the bas ...
genchem study guide test_4a
... C Subdivision of energy level; the numeric value of energy level is equal to the total number of these in that energy level D Empty Bus Seat Rule; electrons occupy equal‐ energy orbitals so that a maximum number of unpaired electrons results E when an electron jumps from one energy level posit ...
... C Subdivision of energy level; the numeric value of energy level is equal to the total number of these in that energy level D Empty Bus Seat Rule; electrons occupy equal‐ energy orbitals so that a maximum number of unpaired electrons results E when an electron jumps from one energy level posit ...
Photo Acoustic Effect And it`s usage for spectroscopy
... depend on transmission or reflection of the light beam – can work with opaque materials, higher immunity to scattering effects May work in various wavelengths Signal depends on various characteristics of medium in addition to absorption (heat capacity, acoustic velocity) that may be used to impr ...
... depend on transmission or reflection of the light beam – can work with opaque materials, higher immunity to scattering effects May work in various wavelengths Signal depends on various characteristics of medium in addition to absorption (heat capacity, acoustic velocity) that may be used to impr ...
1 - kurtniedenzu
... b. Stephen Jay Gould c. Throckmorton P. Guildersleeve d. Ernest B. Rutherford 15. Which numbered arrow in the diagram below gives the best indicator of the time at which the particle model of the atom became generally accepted by chemists and physicists? ...
... b. Stephen Jay Gould c. Throckmorton P. Guildersleeve d. Ernest B. Rutherford 15. Which numbered arrow in the diagram below gives the best indicator of the time at which the particle model of the atom became generally accepted by chemists and physicists? ...
HALL EFFECT IN THIN FILMS When a current
... In order to obtain the resistivity ρ, first measure the resistance between V1 and V2 with the HP DVM using the 4-wire method. Why is it crucial to use the 4-wire method as opposed to the 2wire method? The width of the sample w, and the distance between V1 and V2 can be measured using the travelling ...
... In order to obtain the resistivity ρ, first measure the resistance between V1 and V2 with the HP DVM using the 4-wire method. Why is it crucial to use the 4-wire method as opposed to the 2wire method? The width of the sample w, and the distance between V1 and V2 can be measured using the travelling ...
CHM1045 General Chemistry and Qualitative Analysis
... Relating the type of bond with the electronegativity differences of the elements involved in bonding. Comparing and contrasting the differences between ionic and covalent bonding. Writing the Lewis electron dot structure of elements, ions, ionic compounds, and covalent compounds. Recognizing excepti ...
... Relating the type of bond with the electronegativity differences of the elements involved in bonding. Comparing and contrasting the differences between ionic and covalent bonding. Writing the Lewis electron dot structure of elements, ions, ionic compounds, and covalent compounds. Recognizing excepti ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.