Cumulative Review, entire quarter
... 5. Redistribute the electrons so that every atom larger than boron is surrounded by an octet of electrons. For this purpose, the bonds around the atoms count for both atoms since the bonding pair is around both of the atoms. If necessary, atoms below the second period can accomodate more than eight ...
... 5. Redistribute the electrons so that every atom larger than boron is surrounded by an octet of electrons. For this purpose, the bonds around the atoms count for both atoms since the bonding pair is around both of the atoms. If necessary, atoms below the second period can accomodate more than eight ...
The Onset of Matter-Wave Amplification in a Superradiant Bose
... The former is resonant, and the latter is detuned by the two-photon recoil shift ␦ ⫽ ⌬E/ប. This model predicts a suppression of the gain by a factor of (2␦/⌫)⫺2 ⬀ (␦)⫺2. With the use of a coherent model based on driven two-level systems, we find a suppression by a factor of 12(␦)⫺2, which has the ...
... The former is resonant, and the latter is detuned by the two-photon recoil shift ␦ ⫽ ⌬E/ប. This model predicts a suppression of the gain by a factor of (2␦/⌫)⫺2 ⬀ (␦)⫺2. With the use of a coherent model based on driven two-level systems, we find a suppression by a factor of 12(␦)⫺2, which has the ...
1st Semester Exam in High School Chemistry
... 42. Carbon tetrachloride is a solvent which is used as a refrigerant and also as a cleaning agent. Prior to the 1950s, carbon tetrachloride was manufactured by the chlorination of carbon disulfide: CS2 + 3 Cl2 → CCl4 + S2Cl2 but now it is mainly produced from methane: CH4 + 4 Cl2 → CCl4 + 4 HCl How ...
... 42. Carbon tetrachloride is a solvent which is used as a refrigerant and also as a cleaning agent. Prior to the 1950s, carbon tetrachloride was manufactured by the chlorination of carbon disulfide: CS2 + 3 Cl2 → CCl4 + S2Cl2 but now it is mainly produced from methane: CH4 + 4 Cl2 → CCl4 + 4 HCl How ...
Stimulated Raman Spectroscopy 1 1. Introduction
... a cell that was connected to a listening tube. When the sunlight was repeatedly blocked and unblocked, sound could be heard through the listening tube at the sunlight chopping frequency. The technique saw few applications until about 1968 when a rise in its use began due to the availability of laser ...
... a cell that was connected to a listening tube. When the sunlight was repeatedly blocked and unblocked, sound could be heard through the listening tube at the sunlight chopping frequency. The technique saw few applications until about 1968 when a rise in its use began due to the availability of laser ...
ENT145/3 Materials Engineering Tutorial 1 (Answer) 1. Why is it
... (b) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (c) Two important refinements resulting from the wave-mechanical atomic model are (1) that ele ...
... (b) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (c) Two important refinements resulting from the wave-mechanical atomic model are (1) that ele ...
electrons - TAMU Chemistry
... It is impossible to determine simultaneously both the position and momentum of an electron (or any other small particle). ...
... It is impossible to determine simultaneously both the position and momentum of an electron (or any other small particle). ...
Set 9 - STEMwomen.org
... (d) Calculate the average z component of the electron’s orbital angular momentum for this ground state. (e) Does Lz commute with the hamiltonian for a hydrogen-like atom ? Show whether the z component of the electron’s orbital angular momentum in this hydrogen-like atom is a constant of the motion. ...
... (d) Calculate the average z component of the electron’s orbital angular momentum for this ground state. (e) Does Lz commute with the hamiltonian for a hydrogen-like atom ? Show whether the z component of the electron’s orbital angular momentum in this hydrogen-like atom is a constant of the motion. ...
Draw atomic models showing the appropriate number of electrons
... 2. Type of bond that forms between two atoms of different electronegativities where electrons are unevenly shared 3. The electrical force of attraction that holds ions of opposite charge together 4. A chemical bond in which atoms are held together by their mutual attraction for two electrons they sh ...
... 2. Type of bond that forms between two atoms of different electronegativities where electrons are unevenly shared 3. The electrical force of attraction that holds ions of opposite charge together 4. A chemical bond in which atoms are held together by their mutual attraction for two electrons they sh ...
Rotation ,vibration, electronic spectra
... • We can learn about molecules by studying how molecules absorb, emit, and scatter electromagnetic radiation. • From the equipartition theorem, the N2 molecule may be thought of as two N atoms held together with a massless, rigid rod (rigid rotator model). • In a purely rotational system, the kineti ...
... • We can learn about molecules by studying how molecules absorb, emit, and scatter electromagnetic radiation. • From the equipartition theorem, the N2 molecule may be thought of as two N atoms held together with a massless, rigid rod (rigid rotator model). • In a purely rotational system, the kineti ...
Atomic Absorption Spectrometry
... radiation of the lines of the analyte are produced by the lamp; the presence of the atomic state of the analyte in the flame attenuates or reduces the intensity of the radiation. The absorbance is proportional to the concentration of the analyte, similar to ...
... radiation of the lines of the analyte are produced by the lamp; the presence of the atomic state of the analyte in the flame attenuates or reduces the intensity of the radiation. The absorbance is proportional to the concentration of the analyte, similar to ...
University of Washington Department of Chemistry Chemistry 453
... The total angular momentum L is related to its x, y, and z components by: L2 = L2X + L2Y + L2Z . Therefore in classical mechanics a freely rotating rigid, linear molecule like CO has its angular momentum vector trace out a sphere. In quantum mechanics, because L is quantized according to L = ( + 1) ...
... The total angular momentum L is related to its x, y, and z components by: L2 = L2X + L2Y + L2Z . Therefore in classical mechanics a freely rotating rigid, linear molecule like CO has its angular momentum vector trace out a sphere. In quantum mechanics, because L is quantized according to L = ( + 1) ...
Atomic and Molecular Structure
... Atomic and Molecular Structure The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of ...
... Atomic and Molecular Structure The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of ...
Physical Chemistry II – Exam 1 SOLUTIONS
... Quantization is the idea that a physical property may take only certain allowed (discrete) values. Such properties would be predicted by classical theories to be continuous; that is, the property could take any value. A variety of systems could be used to demonstrate quantization of a physical prope ...
... Quantization is the idea that a physical property may take only certain allowed (discrete) values. Such properties would be predicted by classical theories to be continuous; that is, the property could take any value. A variety of systems could be used to demonstrate quantization of a physical prope ...
8.P.1.1Homework for Website
... 13. Which BEST explains how atoms combine to form compounds? A. The atoms in the compound share electrons B. The atoms in the compound share neutrons C. The atoms in the compound share protons 14. The compound propane has three carbon atoms and eight hydrogen atoms. What is the chemical formula of p ...
... 13. Which BEST explains how atoms combine to form compounds? A. The atoms in the compound share electrons B. The atoms in the compound share neutrons C. The atoms in the compound share protons 14. The compound propane has three carbon atoms and eight hydrogen atoms. What is the chemical formula of p ...
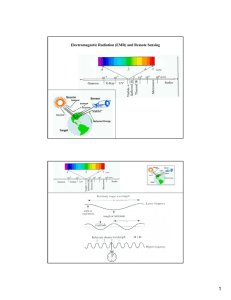
Electromagnetic Radiation (EMR) and Remote Sensing
... Nonselective scattering: Happened when the lower atmosphere contains suspended aerosols. The aerosols should have diameters at least 10 times larger than the wavelengths under consideration. The important scattering agents include large particles of smoke, water vapor, water droplets, ice crystals ...
... Nonselective scattering: Happened when the lower atmosphere contains suspended aerosols. The aerosols should have diameters at least 10 times larger than the wavelengths under consideration. The important scattering agents include large particles of smoke, water vapor, water droplets, ice crystals ...
IOSR Journal of Applied Physics (IOSR-JAP)
... Abstract: The determination of atmospheric temperature needs using complex and expensive technology. This requires a new approach for temperatures determination by using simple and cheap technology .To perform this task an attempt was made in this research to relate temperature change to the change ...
... Abstract: The determination of atmospheric temperature needs using complex and expensive technology. This requires a new approach for temperatures determination by using simple and cheap technology .To perform this task an attempt was made in this research to relate temperature change to the change ...
Resource for Final Exam Prep
... So, E = hc/ 1 nm = 1 10-9 m Photoelectric effect kinetic energy of ejected electron Uncertainty principle you can estimate accurately the position and the momentum of an electron at the same time Quantum numbers (n, l, ml, ms), what do they each define? Value of l for s, p, d and f orbitals E ...
... So, E = hc/ 1 nm = 1 10-9 m Photoelectric effect kinetic energy of ejected electron Uncertainty principle you can estimate accurately the position and the momentum of an electron at the same time Quantum numbers (n, l, ml, ms), what do they each define? Value of l for s, p, d and f orbitals E ...
A.Lobko, Laser acceleration of electrons and ions: principles, issues
... heats more and more electrons to relativistic energies. Provided, the target is thin enough and has not blown apart under the irradiation of the laser pedestal, the laser will eventually promote all electrons within the focal volume to hot electrons and turn the target relativistically transparent. ...
... heats more and more electrons to relativistic energies. Provided, the target is thin enough and has not blown apart under the irradiation of the laser pedestal, the laser will eventually promote all electrons within the focal volume to hot electrons and turn the target relativistically transparent. ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.