GCSE_C2_Revision_+_Exam_Questions
... Substances that consist of simple molecules have only weak forces between the molecules (intermolecular forces). It is these intermolecular forces that are overcome, not the covalent bonds, when the substance melts or boils. Substances that consist of simple molecules do not conduct electricity beca ...
... Substances that consist of simple molecules have only weak forces between the molecules (intermolecular forces). It is these intermolecular forces that are overcome, not the covalent bonds, when the substance melts or boils. Substances that consist of simple molecules do not conduct electricity beca ...
PHYSICAL SETTING CHEMISTRY
... (1) The activation energy of the forward reaction must equal the activation energy of the reverse reaction. (2) The rate of the forward reaction must equal the rate of the reverse reaction. (3) The concentration of the reactants must equal the concentration of the products. (4) The potential energy ...
... (1) The activation energy of the forward reaction must equal the activation energy of the reverse reaction. (2) The rate of the forward reaction must equal the rate of the reverse reaction. (3) The concentration of the reactants must equal the concentration of the products. (4) The potential energy ...
Name: Per: Date: Unit 1. Materials: Formulating Matter B. Periodic
... smallest whole numbers! [For example, Ca2O2 simplifies to become CaO] Ions a. Al3+ + O2-– ...
... smallest whole numbers! [For example, Ca2O2 simplifies to become CaO] Ions a. Al3+ + O2-– ...
Pre-AP Chemistry - Simple Rules for Electron Exchange Simple
... Tracking how they change as each chemical species goes from reactants to products helps us keep track of which species loses and which species gains electrons. You will note that oxidation numbers (or “oxidation states” for groups of like atoms) are similar, but not identical to, formal ionic charge ...
... Tracking how they change as each chemical species goes from reactants to products helps us keep track of which species loses and which species gains electrons. You will note that oxidation numbers (or “oxidation states” for groups of like atoms) are similar, but not identical to, formal ionic charge ...
1 2016-17 Honors Chemistry Review for the Final Exam Each unit
... b) This is a product made from a metal and a nonmetal. However, the bonds have a more covalent character to them than ionic. Name this product using both the ionic and covalent nomenclature systems. c) ...
... b) This is a product made from a metal and a nonmetal. However, the bonds have a more covalent character to them than ionic. Name this product using both the ionic and covalent nomenclature systems. c) ...
Title of PAPER - Department of Physics and Astronomy
... can be reinforced or other materials can be used with a higher melting point to increase the durability of the mirror. Conclusion To summarise, if conductivity and scattering is ignored, lasers with a wavelength of 500 nm and lower will melt a mirror with silver as its optical coating. Different mat ...
... can be reinforced or other materials can be used with a higher melting point to increase the durability of the mirror. Conclusion To summarise, if conductivity and scattering is ignored, lasers with a wavelength of 500 nm and lower will melt a mirror with silver as its optical coating. Different mat ...
Powerpoint
... physically, because the species of the two half-cells react directly without electrons going through the external circuit. ...
... physically, because the species of the two half-cells react directly without electrons going through the external circuit. ...
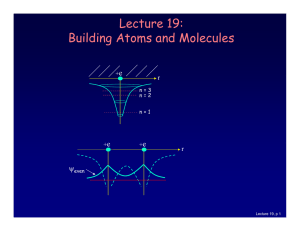
Lecture 19: Building Atoms and Molecules
... Example: Nuclear Spin and MRI Magnetic resonance imaging (MRI) depends on the absorption of electromagnetic radiation by the nuclear spin of the hydrogen atoms in our bodies. The nucleus is a proton with spin ½, so in a magnetic field B there are two energy states. The proton’s magnetic moment is µ ...
... Example: Nuclear Spin and MRI Magnetic resonance imaging (MRI) depends on the absorption of electromagnetic radiation by the nuclear spin of the hydrogen atoms in our bodies. The nucleus is a proton with spin ½, so in a magnetic field B there are two energy states. The proton’s magnetic moment is µ ...
Chapter 2 BIO 100 Chemistry
... •If electrons in a covalent bond are shared equally, it’s a nonpolar covalent bond. • Covalent bond between two atoms of the same element is always nonpolar. •A covalent bond between atoms that have similar electronegativities is also nonpolar. •Because carbon and hydrogen do not differ greatly in e ...
... •If electrons in a covalent bond are shared equally, it’s a nonpolar covalent bond. • Covalent bond between two atoms of the same element is always nonpolar. •A covalent bond between atoms that have similar electronegativities is also nonpolar. •Because carbon and hydrogen do not differ greatly in e ...
Semester 2 Final Exam
... (D) 33.3 g 6. The units for heat are: (A) J (B) J/g (C) J/g·°C (D) J/°C 7. 20.0 gram samples of each of the following metals are originally at 10°C. They are heated evenly for ten minutes. Which metal will have the highest temperature at the end? (A) Aluminum (c = 0.900 J/g·°C) (B) Copper (c = 0.386 ...
... (D) 33.3 g 6. The units for heat are: (A) J (B) J/g (C) J/g·°C (D) J/°C 7. 20.0 gram samples of each of the following metals are originally at 10°C. They are heated evenly for ten minutes. Which metal will have the highest temperature at the end? (A) Aluminum (c = 0.900 J/g·°C) (B) Copper (c = 0.386 ...
the Quantifying Scatter PDF
... Scatter signals can be easily quantified as scattered light power per unit solid angle (in watts per steradian); however, in order to make the results more meaningful, these signals are usually normalized, in some fashion, by the light incident on the scatter source. The three ways commonly employed ...
... Scatter signals can be easily quantified as scattered light power per unit solid angle (in watts per steradian); however, in order to make the results more meaningful, these signals are usually normalized, in some fashion, by the light incident on the scatter source. The three ways commonly employed ...
Tugas Kimia Umum
... force and electric forces, in one drop of oil that in the plat of electrode. Knowing the value of electric area, the electric capacity in oil that was fallen can be determine. And then repeat this experiment, he find the value that was measure always multiple from the same number. And the finally, h ...
... force and electric forces, in one drop of oil that in the plat of electrode. Knowing the value of electric area, the electric capacity in oil that was fallen can be determine. And then repeat this experiment, he find the value that was measure always multiple from the same number. And the finally, h ...
Chemistry a material science!
... kind of particle such as an atom or molecule is a pure substance or simply a substance. ...
... kind of particle such as an atom or molecule is a pure substance or simply a substance. ...
Manifestation of classical phase in a single spontaneously emitted
... Abstract: We note a manifestation of the classical phase of a traveling wave in a multimode quantized field. We study spontaneous emission, scattering, and re-absorption by two-level atoms in a one-dimensional optical cavity, and observe classical phase information in the complex quantum amplitude f ...
... Abstract: We note a manifestation of the classical phase of a traveling wave in a multimode quantized field. We study spontaneous emission, scattering, and re-absorption by two-level atoms in a one-dimensional optical cavity, and observe classical phase information in the complex quantum amplitude f ...
1. Select the correct statement about subatomic particles. a
... e. It represents a molecule made of 1 carbon atom, 2 hydrogen atoms, and 6 oxygen atoms. 24. Select the correct statement about the formula K2O. a. It represents a molecule of potassium oxide. b. It represents a substance composed of potassium atoms and oxygen atoms. c. It represents a substance con ...
... e. It represents a molecule made of 1 carbon atom, 2 hydrogen atoms, and 6 oxygen atoms. 24. Select the correct statement about the formula K2O. a. It represents a molecule of potassium oxide. b. It represents a substance composed of potassium atoms and oxygen atoms. c. It represents a substance con ...
Document
... 10. Compared to the charge of a proton, the charge of an electron has a. A greater magnitude and the same sign b. A greater magnitude and the opposite sign c. The same magnitude and the same sign d. The same magnitude and the opposite sign 11. Which phrase describes an atom? a. A negatively charged ...
... 10. Compared to the charge of a proton, the charge of an electron has a. A greater magnitude and the same sign b. A greater magnitude and the opposite sign c. The same magnitude and the same sign d. The same magnitude and the opposite sign 11. Which phrase describes an atom? a. A negatively charged ...
chapter 2
... distillation a process used to separate components of a mixture using differences in boiling points ...
... distillation a process used to separate components of a mixture using differences in boiling points ...
Chapt3
... Ionic Compounds -- Ionic Bonding -- electron transfer result from transfer of one or more electrons from one atom to another to yield oppositely-charged particles called ions cation = positive ion ...
... Ionic Compounds -- Ionic Bonding -- electron transfer result from transfer of one or more electrons from one atom to another to yield oppositely-charged particles called ions cation = positive ion ...
Fall Exam 4 - Chemistry - University of Kentucky
... He theorized that atoms combine in simple, whole-number ratios to form compounds. He used the oil drop experiment to measure the charge of the electron. ...
... He theorized that atoms combine in simple, whole-number ratios to form compounds. He used the oil drop experiment to measure the charge of the electron. ...
Early Atomic Models
... Demitri Mendeleev used this theory when he constructed the first working periodic table. ...
... Demitri Mendeleev used this theory when he constructed the first working periodic table. ...
Final Velocity (V f )
... Protons – positive charge, found in the nucleus of an atom, has mass, # located in the bottom left corner of the symbol Neutrons – no charge, neutral, found in the nucleus, has mass (neutrons = mass – protons) Electrons – negative charge, surround the nucleus in energy levels, very small, very fast, ...
... Protons – positive charge, found in the nucleus of an atom, has mass, # located in the bottom left corner of the symbol Neutrons – no charge, neutral, found in the nucleus, has mass (neutrons = mass – protons) Electrons – negative charge, surround the nucleus in energy levels, very small, very fast, ...
experiment 8 radioactive decay of nuclei
... Indium metal (49In, atomic number of 49) as found on the surface of the earth is 95.72 % mass 115 and 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where th ...
... Indium metal (49In, atomic number of 49) as found on the surface of the earth is 95.72 % mass 115 and 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where th ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.