Atom:Mole TEST05key
... 11. The bright-light spectra observed for different elements result from 1) collisions between electrons of different energies. 2) changes within the nucleus of the atom. 3) electrons changing directly into energy. 4) electrons moving to lower energy levels. ANS: 4 12. When Rutherford bombarded gold ...
... 11. The bright-light spectra observed for different elements result from 1) collisions between electrons of different energies. 2) changes within the nucleus of the atom. 3) electrons changing directly into energy. 4) electrons moving to lower energy levels. ANS: 4 12. When Rutherford bombarded gold ...
Chapter 2. The Chemical Context of Life
... Atoms have a Nucleus which contain Protons & Neutrons. • Protons are Positively Charged and have a mass ...
... Atoms have a Nucleus which contain Protons & Neutrons. • Protons are Positively Charged and have a mass ...
Inelastic Light Scattering by Elementary Excitations of the
... (2DEG) in semiconductor nanostructures have uncovered remarkable new phenomena associated with electronic correlations in reduced dimensions [1]. Coulomb interactions manifest themselves in the spectrum of elementary excitations of the electron gas, which can be measured by inelastic light scatterin ...
... (2DEG) in semiconductor nanostructures have uncovered remarkable new phenomena associated with electronic correlations in reduced dimensions [1]. Coulomb interactions manifest themselves in the spectrum of elementary excitations of the electron gas, which can be measured by inelastic light scatterin ...
Measuring cellular structure at submicrometer scale with light
... diluting the stock suspension with de-ionized and distilled water to obtain the required optical density. Fig. 3 summarizes the experimental results obtained for polystyrene particles. To demonstrate the symmetry of the A/LSS scattering patterns, the parallel-polarized component of the backscattered ...
... diluting the stock suspension with de-ionized and distilled water to obtain the required optical density. Fig. 3 summarizes the experimental results obtained for polystyrene particles. To demonstrate the symmetry of the A/LSS scattering patterns, the parallel-polarized component of the backscattered ...
Document
... •Each subshell is designated by a letter: • l = 0 indicates an s subshell. • l = 1 indicates a p subshell. • l = 2 indicates a d subshell. • l = 3 indicates an f subshell. • Subshells are named by using the n value and the letter designation. The subshell with n = 2 and l = 0 is called the 2s subsh ...
... •Each subshell is designated by a letter: • l = 0 indicates an s subshell. • l = 1 indicates a p subshell. • l = 2 indicates a d subshell. • l = 3 indicates an f subshell. • Subshells are named by using the n value and the letter designation. The subshell with n = 2 and l = 0 is called the 2s subsh ...
Orbitals
... that the electron of hydrogen circles the nucleus in allowed orbits. That is, the electron is in its ground state in an orbit closest to the nucleus. As the atom becomes excited, the electron is promoted to an orbit further away from the nucleus. ...
... that the electron of hydrogen circles the nucleus in allowed orbits. That is, the electron is in its ground state in an orbit closest to the nucleus. As the atom becomes excited, the electron is promoted to an orbit further away from the nucleus. ...
Webquest Review - Harrison High School
... polar and will have dipole-dipole interactions between molecules. That means CH3Cl will be harder to boil than CO2. H2O has H bonded to O and it’s polar. When you have species with H bonded to F, O, or N, hydrogen “bond”ing sets up between the molecules. Hydrogen bonding is the strongest intermolecu ...
... polar and will have dipole-dipole interactions between molecules. That means CH3Cl will be harder to boil than CO2. H2O has H bonded to O and it’s polar. When you have species with H bonded to F, O, or N, hydrogen “bond”ing sets up between the molecules. Hydrogen bonding is the strongest intermolecu ...
che-20028 QC lecture 1 - Rob Jackson`s Website
... How the experiment is performed • Using a variable frequency light source, shine light onto a metal surface. • Determine the light frequency which causes electrons to be emitted. • Measure the energy of the emitted electrons, by applying a voltage across the cell in the opposite direction to balanc ...
... How the experiment is performed • Using a variable frequency light source, shine light onto a metal surface. • Determine the light frequency which causes electrons to be emitted. • Measure the energy of the emitted electrons, by applying a voltage across the cell in the opposite direction to balanc ...
Name ______Mr. Perfect_______________________________
... Name ______Mr. Perfect_______________________________ Date ____Sp 09_____ 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
... Name ______Mr. Perfect_______________________________ Date ____Sp 09_____ 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
OKEMOS PUBLIC SCHOOLS
... Electrons jump to higher energy levels when they (lose/gain) energy. When they fall back to their ground state, energy is (gained/released). Because the difference in energy levels is constant, the amount of energy is a fixed amount. This results in photons of light which have a fixed _frequency__ a ...
... Electrons jump to higher energy levels when they (lose/gain) energy. When they fall back to their ground state, energy is (gained/released). Because the difference in energy levels is constant, the amount of energy is a fixed amount. This results in photons of light which have a fixed _frequency__ a ...
CHEMISTRY FALL FINAL PRACTICE 2016
... c. Find the mass number of the most common isotope of an element _________ d. Get how many neutrons the most common isotope has _________ e. How many valence electrons an element has _________ f. What is its charge/oxidation number for the atom _________ g. Know what ion is forms/write the ion _____ ...
... c. Find the mass number of the most common isotope of an element _________ d. Get how many neutrons the most common isotope has _________ e. How many valence electrons an element has _________ f. What is its charge/oxidation number for the atom _________ g. Know what ion is forms/write the ion _____ ...
Laser and its applications
... diminished and for those atoms moving away it is increased in accordance with Doppler’s principle. When we have a moving source sending out waves continuously it moves. The velocity of the waves is often not changed but the wavelength and frequency as noted by ...
... diminished and for those atoms moving away it is increased in accordance with Doppler’s principle. When we have a moving source sending out waves continuously it moves. The velocity of the waves is often not changed but the wavelength and frequency as noted by ...
Hybrid Dielectric/Surface Plasmon Polariton Waveguide P. David Flammer
... waveguide that can be used in either a single mode, single polarization waveguide, or in a multi-mode. When used as a single mode, the devise allows for control of propagation and confinement. Gratings may be used for coupling light into and out of the modes or for use as mirrors in the mode. When u ...
... waveguide that can be used in either a single mode, single polarization waveguide, or in a multi-mode. When used as a single mode, the devise allows for control of propagation and confinement. Gratings may be used for coupling light into and out of the modes or for use as mirrors in the mode. When u ...
Science 1206 Unit 3 Part 1
... These ions are listed on your periodic table of ions but this list is not exhausted, i.e. There are more polyatomic ions than what’s listed. ...
... These ions are listed on your periodic table of ions but this list is not exhausted, i.e. There are more polyatomic ions than what’s listed. ...
... 632.8 nm. Never look directly at a laser beam nor permit anyone else to do so! Exposure to the direct or reflected beam for more than a few seconds will cause serious eye damage. Do not pick up the lasers and shine them around the room. If these simple precautions are taken then there will be no ris ...
Cold interactions between an Yb ion and a Li atom
... Before presenting our potential-energy curves, and permanent and transition dipole moments, we first compare the computed atomic results to the best available experimental data. Our predicted position of the nonrelativistic 2 P state of the Li atom is 14 910 cm−1 , to be compared with the experiment ...
... Before presenting our potential-energy curves, and permanent and transition dipole moments, we first compare the computed atomic results to the best available experimental data. Our predicted position of the nonrelativistic 2 P state of the Li atom is 14 910 cm−1 , to be compared with the experiment ...
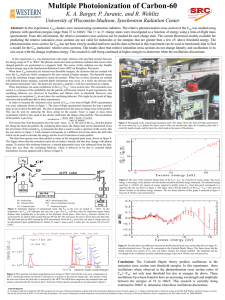
Figure 4 - University of Wisconsin–Madison
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
Chemistry - Halifax County Public Schools
... The mass of zinc used and hydrogen production are directly proportional. The mass of zinc used and hydrogen production are inversely proportional. The mass of zinc used and hydrogen production are related, but not proportional. The mass of zinc used and hydrogen production are unrelated. ...
... The mass of zinc used and hydrogen production are directly proportional. The mass of zinc used and hydrogen production are inversely proportional. The mass of zinc used and hydrogen production are related, but not proportional. The mass of zinc used and hydrogen production are unrelated. ...
Chapter notes Class: IX Chapter Name: Atoms and molecules Top
... Symbol of the more metallic element is written first in formula Number of atoms of each of the constituent element present in the molecule is indicated by subscript When either of the ions or both the ions are polyatomic and their valency is more than 1, we enclose the polyatomic ions in brack ...
... Symbol of the more metallic element is written first in formula Number of atoms of each of the constituent element present in the molecule is indicated by subscript When either of the ions or both the ions are polyatomic and their valency is more than 1, we enclose the polyatomic ions in brack ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.