Scanning Tunneling Microscope
... What an STM measures?------Local density of states Each plane represents a different value of the tip-sample V, and the lateral position on the plane gives the x,y position of the tip. Filled states are given in red. The plane at the Fermi energy (V=0) is shown in blue. ...
... What an STM measures?------Local density of states Each plane represents a different value of the tip-sample V, and the lateral position on the plane gives the x,y position of the tip. Filled states are given in red. The plane at the Fermi energy (V=0) is shown in blue. ...
Atom Smallest particle of an element having the same chemical
... Rutherford’s Nuclear Atom: Atom: heavy positively charged small region soft negatively charged large region. #protons = # electrons; atom overall neutral. Nucleus: protons + neutrons virtually all mass in nucleus. Soft sphere. ...
... Rutherford’s Nuclear Atom: Atom: heavy positively charged small region soft negatively charged large region. #protons = # electrons; atom overall neutral. Nucleus: protons + neutrons virtually all mass in nucleus. Soft sphere. ...
Balancing Chemical Equations
... compounds. Therefore you add another to the front of the compound. Since the compounds cannot be separated, you cannot just add a two in front of the oxygen atom only, it has to be in front of the whole compound. ...
... compounds. Therefore you add another to the front of the compound. Since the compounds cannot be separated, you cannot just add a two in front of the oxygen atom only, it has to be in front of the whole compound. ...
Slow Photoelectron Imaging
... was possible at wavelengths #651.76 nm. Under our experimental conditions the Stark structure was not resolved and the laser excited an incoherent superposition of Stark and continuum states. The imaging detector consists of an extraction region containing two electrodes which create the aforementio ...
... was possible at wavelengths #651.76 nm. Under our experimental conditions the Stark structure was not resolved and the laser excited an incoherent superposition of Stark and continuum states. The imaging detector consists of an extraction region containing two electrodes which create the aforementio ...
Grade 11 Chemistry Exam Review
... Element X consists of 30.00% of an isotope with mass 24.02 u and 70.00% of an isotope with mass 26.10 u. The average atomic mass of X is a) 24.64 u. b) 25.06 u. c) 25.48 u. d) 50.12 u ...
... Element X consists of 30.00% of an isotope with mass 24.02 u and 70.00% of an isotope with mass 26.10 u. The average atomic mass of X is a) 24.64 u. b) 25.06 u. c) 25.48 u. d) 50.12 u ...
Name: 1) At 1 atmosphere and 298 K, 1 mole of H O(l) molecules
... They're baaack... a splash from the past! Fizzies instant sparkling drink tablets, popular in the 1950's and 1960's, are now back on the market. What sets them apart from other powdered drinks is that they bubble and fizz when placed in water, forming an instant carbonated beverage. The fizz in Fizz ...
... They're baaack... a splash from the past! Fizzies instant sparkling drink tablets, popular in the 1950's and 1960's, are now back on the market. What sets them apart from other powdered drinks is that they bubble and fizz when placed in water, forming an instant carbonated beverage. The fizz in Fizz ...
Chemistry Final Exam Review 2006-2007
... How many electrons, protons, and neutrons does an ion of 9 Be have? a. e- = 2, p+= 4, n0= 9 b. e- = 2, p+= 4, n0= 5 c. e- = 4, p+= 4, n0= 5 d. e- = 4, p+= 2, n0= 5 How many electrons, protons, and neutrons does an ion of 32 P have? a. e- = 15, p+= 15, n0= 17 b. e- = 15, p+= 17, n0= 15 c. e- = 18, p+ ...
... How many electrons, protons, and neutrons does an ion of 9 Be have? a. e- = 2, p+= 4, n0= 9 b. e- = 2, p+= 4, n0= 5 c. e- = 4, p+= 4, n0= 5 d. e- = 4, p+= 2, n0= 5 How many electrons, protons, and neutrons does an ion of 32 P have? a. e- = 15, p+= 15, n0= 17 b. e- = 15, p+= 17, n0= 15 c. e- = 18, p+ ...
In the Classroom
... the immediate cause of the molecular ground state is a sharp increase in electron kinetic energy. • The amount of electron density transferred to the bonding region is greatly overstated, sometimes implying that a pair of electrons is shared in the space between two nuclei rather than by two nuclei. ...
... the immediate cause of the molecular ground state is a sharp increase in electron kinetic energy. • The amount of electron density transferred to the bonding region is greatly overstated, sometimes implying that a pair of electrons is shared in the space between two nuclei rather than by two nuclei. ...
Atoms
... A white light consists of photons with all frequencies in the visible region, and it has a continuous spectrum, with intensities varying continuously as a function of the frequency. An object with various amounts of energies for light emission, such as a hot solid, emits a white light beam. A combin ...
... A white light consists of photons with all frequencies in the visible region, and it has a continuous spectrum, with intensities varying continuously as a function of the frequency. An object with various amounts of energies for light emission, such as a hot solid, emits a white light beam. A combin ...
Chemical Equations
... formulas for the reactants (on the left of the arrow) and the products (on the right of the arrow). C. The law of conservation of mass and energy must be satisfied. Therefore the same number of atoms of each element must appear on each side of a correct chemical equation. ...
... formulas for the reactants (on the left of the arrow) and the products (on the right of the arrow). C. The law of conservation of mass and energy must be satisfied. Therefore the same number of atoms of each element must appear on each side of a correct chemical equation. ...
Earth Materials
... Rocks in the Earth's crust and mantle are made up of mineral assemblages with chemical compounds, elements, molecular bonds which are formed from ordered atomic structures. ...
... Rocks in the Earth's crust and mantle are made up of mineral assemblages with chemical compounds, elements, molecular bonds which are formed from ordered atomic structures. ...
Ch1 Mod Review.WXP
... The Compton effect, the photoelectric effect, and the measured blackbody radiation spectrum clearly demonstrated the “particle nature” of radiation in contrast to the previously accepted and demonstrated “wave nature” of radiation. This led to a wave-particle duality, i.e., a dual description of the ...
... The Compton effect, the photoelectric effect, and the measured blackbody radiation spectrum clearly demonstrated the “particle nature” of radiation in contrast to the previously accepted and demonstrated “wave nature” of radiation. This led to a wave-particle duality, i.e., a dual description of the ...
Sections 6.4 - 6.5
... Through the use of lead piping the Romans found out that Pb(OAc)2 tastes very sweet and used to add it to their wine. Some historians claim that this habit contributed to the collapse of their empire – they were all a little dumber than they should have been … ...
... Through the use of lead piping the Romans found out that Pb(OAc)2 tastes very sweet and used to add it to their wine. Some historians claim that this habit contributed to the collapse of their empire – they were all a little dumber than they should have been … ...
Practice problems for chapter 1, 3 and 5 1) A small amount of salt
... Practice problems for chapter 1, 3 and 5 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substa ...
... Practice problems for chapter 1, 3 and 5 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substa ...
Chapter 1: Matter, Measurement and Problem Solving
... minimum number of digits needed to write a given value without loss of ...
... minimum number of digits needed to write a given value without loss of ...
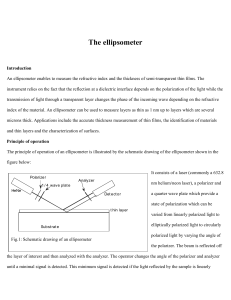
ellip
... An ellipsometer enables to measure the refractive index and the thickness of semi-transparent thin films. The instrument relies on the fact that the reflection at a dielectric interface depends on the polarization of the light while the transmission of light through a transparent layer changes the p ...
... An ellipsometer enables to measure the refractive index and the thickness of semi-transparent thin films. The instrument relies on the fact that the reflection at a dielectric interface depends on the polarization of the light while the transmission of light through a transparent layer changes the p ...
ZCT 104 Test II solution
... II(T) Frank-Hertz experimental result is consistent with the results suggested by the line spectra III (T) The predictions of the quantum theory for the behaviour of any physical system must correspond to the prediction of classical physics in the limit in which the quantum number specifying the sta ...
... II(T) Frank-Hertz experimental result is consistent with the results suggested by the line spectra III (T) The predictions of the quantum theory for the behaviour of any physical system must correspond to the prediction of classical physics in the limit in which the quantum number specifying the sta ...
2002 Final Exam for Practice - Department of Chemistry | Oregon
... Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a basic calculator (for example, TI-25X Solar or TI-30XA) if you wish. If you have notes or electronic devices with you, place them in a sealed backpack and p ...
... Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a basic calculator (for example, TI-25X Solar or TI-30XA) if you wish. If you have notes or electronic devices with you, place them in a sealed backpack and p ...
Periodic Table Review Key
... B, H 15. Which element is in group 2? C 16. Which element is in period 6? H 17. Which element has an atomic number of 12? C 18. Which elements have 5 electron clouds? E,D 19. Which element has 14 protons? G 20. Which element has a combined total of 35 protons and neutrons? 21. Which element has 12 e ...
... B, H 15. Which element is in group 2? C 16. Which element is in period 6? H 17. Which element has an atomic number of 12? C 18. Which elements have 5 electron clouds? E,D 19. Which element has 14 protons? G 20. Which element has a combined total of 35 protons and neutrons? 21. Which element has 12 e ...
Chapter 2 Name___________________________________
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) If an atom of sulfur (atomic number 16) were allowed to react with atoms of hydrogen (atomic number 1), which of the molecules below would be formed? H A) S H B) H S H C) H S H D) E) H S H ...
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) If an atom of sulfur (atomic number 16) were allowed to react with atoms of hydrogen (atomic number 1), which of the molecules below would be formed? H A) S H B) H S H C) H S H D) E) H S H ...
8.P.1.1 Warm-Up Questions for Website
... is made up of one type of atom. B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
... is made up of one type of atom. B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.