Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
Periodic Trends Worksheet Answers Page 1: 1. Rank the following
... 1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, and potassium. O, C, Al, K 2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, and aluminum. Ne, Al, S, O 3. Why does fluorine have a higher ionization energy than iodine? Fluorine ...
... 1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, and potassium. O, C, Al, K 2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, and aluminum. Ne, Al, S, O 3. Why does fluorine have a higher ionization energy than iodine? Fluorine ...
CLASSIFICATION OF THE ELEMENTS
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 11. The representative elements are the Group A elements. ________ 12. Chlorine has the electron configuration 1s22s22p63s23p7. ________ 13. The element in Group 4A, period 3, is g ...
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 11. The representative elements are the Group A elements. ________ 12. Chlorine has the electron configuration 1s22s22p63s23p7. ________ 13. The element in Group 4A, period 3, is g ...
Periodic Trends Student
... • It doesn’t take much energy to pull electrons from alkali metals, since they want to give them away. And it takes lots of energy to pull electrons from noble gases, since they are so happy with their full outer shell. ...
... • It doesn’t take much energy to pull electrons from alkali metals, since they want to give them away. And it takes lots of energy to pull electrons from noble gases, since they are so happy with their full outer shell. ...
4-3 Families of Elements
... ii. The last two periods of the transition metals are placed toward the bottom of the periodic table so that similar elements elsewhere in the table still line up. iii. All elements with atomic numbers greater than 92 are man-made III. Nonmetals a. Carbon is found in three different forms and can al ...
... ii. The last two periods of the transition metals are placed toward the bottom of the periodic table so that similar elements elsewhere in the table still line up. iii. All elements with atomic numbers greater than 92 are man-made III. Nonmetals a. Carbon is found in three different forms and can al ...
ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
Anticipation Guide Before After The periodic table is a collection of
... The periodic table is a collection of all elements known to us. The atomic number is the number of protons in the nucleus. The atomic number is written below the element on the Periodic Table. The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the el ...
... The periodic table is a collection of all elements known to us. The atomic number is the number of protons in the nucleus. The atomic number is written below the element on the Periodic Table. The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the el ...
Periodic Table Notes
... The periodic table as we have it today has not always existed; it developed much in the same way as atomic theory did. In the early 1800’s scientists began looking for ways to classify the elements that had been discovered. ...
... The periodic table as we have it today has not always existed; it developed much in the same way as atomic theory did. In the early 1800’s scientists began looking for ways to classify the elements that had been discovered. ...
Name: Per: _____ Date: ______ Unit 5 Redemption Packet: The
... 34. For which of these properties does lithium have a larger value than potassium? A. First ionization energy B. Atomic radius C. Electronegativity D. Ionic radius 35. Which of the following properties generally increases as we go from the lower left to the upper right of the periodic table? A. Atom ...
... 34. For which of these properties does lithium have a larger value than potassium? A. First ionization energy B. Atomic radius C. Electronegativity D. Ionic radius 35. Which of the following properties generally increases as we go from the lower left to the upper right of the periodic table? A. Atom ...
Elements and the Periodic Table
... Scientists call these allotropes. One example of this is carbon. Depending on how carbon atoms fit together they can form diamond, coal, or graphite. How many elements are there? There are currently 118 known elements. Of these, only 94 are thought to naturally exist on Earth. Families of Elements E ...
... Scientists call these allotropes. One example of this is carbon. Depending on how carbon atoms fit together they can form diamond, coal, or graphite. How many elements are there? There are currently 118 known elements. Of these, only 94 are thought to naturally exist on Earth. Families of Elements E ...
alkaline earth metals
... left of the PT and very reactive • Transition metals—near the center of the PT and include copper, gold, silver, and iron • Rare earth metals– in the top row of the 2 rows of metals show outside the main body of the PT • Two bottom rows—separated from the table to save space. ...
... left of the PT and very reactive • Transition metals—near the center of the PT and include copper, gold, silver, and iron • Rare earth metals– in the top row of the 2 rows of metals show outside the main body of the PT • Two bottom rows—separated from the table to save space. ...
Periodic Table and Trends
... Defined by an atoms tendency to attract electrons in a chemical bond. In a chemical bond, atoms have to share electrons The atom with a higher electronegativity will keep the electrons closer to it. ...
... Defined by an atoms tendency to attract electrons in a chemical bond. In a chemical bond, atoms have to share electrons The atom with a higher electronegativity will keep the electrons closer to it. ...
Groups of the Periodic Table
... Energy Levels and the Periodic Table (pg. 295) 10.Periods represent the number of ______________ ________________ needed to hold the appropriate number of electrons. 11. Where are the outermost electrons in atoms located and why are they important? ...
... Energy Levels and the Periodic Table (pg. 295) 10.Periods represent the number of ______________ ________________ needed to hold the appropriate number of electrons. 11. Where are the outermost electrons in atoms located and why are they important? ...
Periodic Table and Atomic Structure Summary
... Astatine is a radioactive element that does not occur naturally on Earth. Like hydrogen, nitrogen and oxygen, the halogens are diatomic molecules. This means they exist in a molecule of two atoms. This means that we can write a chemical formula for them: F2, Cl2, Br2, I2, At2. ...
... Astatine is a radioactive element that does not occur naturally on Earth. Like hydrogen, nitrogen and oxygen, the halogens are diatomic molecules. This means they exist in a molecule of two atoms. This means that we can write a chemical formula for them: F2, Cl2, Br2, I2, At2. ...
atomic number
... their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur. ...
... their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur. ...
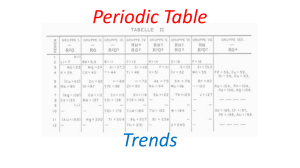
2- Periodic Trends
... • Arranged the 63 elements by atomic mass • Noticed a repetition of properties (periodicity) • Called the pattern of properties “Periodic Law” ...
... • Arranged the 63 elements by atomic mass • Noticed a repetition of properties (periodicity) • Called the pattern of properties “Periodic Law” ...
Periodic Table PP revised 2014
... • Elements in the same group will have similar chemical and physical properties (because they have the same number of valence electrons) ...
... • Elements in the same group will have similar chemical and physical properties (because they have the same number of valence electrons) ...
chapter 4 crossword pre-ap
... 5. Group 2 elements. 10. The number of valence electrons of a noble gas. 15. A reaction that affects the nucleus of an atom. 18. Aluminum has ___ valence electrons. 21. An electron that is found in the outermost shell of an atom and that determines the atom's chemical properties. 22. Gold is in peri ...
... 5. Group 2 elements. 10. The number of valence electrons of a noble gas. 15. A reaction that affects the nucleus of an atom. 18. Aluminum has ___ valence electrons. 21. An electron that is found in the outermost shell of an atom and that determines the atom's chemical properties. 22. Gold is in peri ...
ATOMIC SIZE
... The number of protons and electrons in a neutral atom is equal, but because of the odd shapes of electron orbitals, that increasing the number of protons in the nucleus effectively holds electrons more closely and more tightly to the nucleus. This effect is somewhat diminished in larger atoms, wher ...
... The number of protons and electrons in a neutral atom is equal, but because of the odd shapes of electron orbitals, that increasing the number of protons in the nucleus effectively holds electrons more closely and more tightly to the nucleus. This effect is somewhat diminished in larger atoms, wher ...
Review: Atomic structure/Periodic Table
... John Dalton Niels Bohr Proton electron nucleus orbital atomic mass semiconductor nonmetal ...
... John Dalton Niels Bohr Proton electron nucleus orbital atomic mass semiconductor nonmetal ...
In the space provided, write the letter of the term or phrase that best
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
Periodic Table Powerpoint
... Transition Elements include those elements in the B families. These are the metals you are probably most ...
... Transition Elements include those elements in the B families. These are the metals you are probably most ...
Noble gas

The noble gases make a group of chemical elements with similar properties. Under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six noble gases that occur naturally are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).For the first six periods of the periodic table, the noble gases are exactly the members of group 18 of the periodic table.It is possible that due to relativistic effects, the group 14 element flerovium exhibits some noble-gas-like properties, instead of the group 18 element ununoctium. Noble gases are typically highly unreactive except when under particular extreme conditions. The inertness of noble gases makes them very suitable in applications where reactions are not wanted. For example: argon is used in lightbulbs to prevent the hot tungsten filament from oxidizing; also, helium is breathed by deep-sea divers to prevent oxygen and nitrogen toxicity.The properties of the noble gases can be well explained by modern theories of atomic structure: their outer shell of valence electrons is considered to be ""full"", giving them little tendency to participate in chemical reactions, and it has been possible to prepare only a few hundred noble gas compounds. The melting and boiling points for a given noble gas are close together, differing by less than 10 °C (18 °F); that is, they are liquids over only a small temperature range.Neon, argon, krypton, and xenon are obtained from air in an air separation unit using the methods of liquefaction of gases and fractional distillation. Helium is sourced from natural gas fields which have high concentrations of helium in the natural gas, using cryogenic gas separation techniques, and radon is usually isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds (since those compounds give off alpha particles). Noble gases have several important applications in industries such as lighting, welding, and space exploration. A helium-oxygen breathing gas is often used by deep-sea divers at depths of seawater over 55 m (180 ft) to keep the diver from experiencing oxygen toxemia, the lethal effect of high-pressure oxygen, and nitrogen narcosis, the distracting narcotic effect of the nitrogen in air beyond this partial-pressure threshold. After the risks caused by the flammability of hydrogen became apparent, it was replaced with helium in blimps and balloons.