8492_Chemichal Weapons Production Indicators
... Phosgene is used as an intermediate in the manufacture of many organic chemicals. The largest amount (approximately 80% of world production) is used to produce toluene diisocyanate and other isocyanates. ...
... Phosgene is used as an intermediate in the manufacture of many organic chemicals. The largest amount (approximately 80% of world production) is used to produce toluene diisocyanate and other isocyanates. ...
Ch 8 AP Practice
... (B) H2O (C) CH4 (D) C2H4 (E) PH3 3. The molecule with only one double bond 4. The molecule with the largest dipole moment 5. The molecule that has trigonal pyramidal geometry 53. According to the VSEPR model, the progressive decrease in the bond angles in the series of molecules CH4, NH3, and H2O is ...
... (B) H2O (C) CH4 (D) C2H4 (E) PH3 3. The molecule with only one double bond 4. The molecule with the largest dipole moment 5. The molecule that has trigonal pyramidal geometry 53. According to the VSEPR model, the progressive decrease in the bond angles in the series of molecules CH4, NH3, and H2O is ...
ppt Sc10 Review Notes
... eg) Li(s), Cu(s), Hg(l) nonmetals and hydrogen do not exist as single atoms – flagpole! ...
... eg) Li(s), Cu(s), Hg(l) nonmetals and hydrogen do not exist as single atoms – flagpole! ...
chapter 2 - Scranton Prep Biology
... ' Can form betweenmoleculesor betweendifferent parts of a single large molecule. ' Help stabilizethe three-dimensional shapeof largemolecules(e.g., DNA and proteins). l. Hydrogen bonds Hydrogen bond : Bond formedby the chargeattractionwhen a hydrogenatom covalentlybondedto one electronegative atom i ...
... ' Can form betweenmoleculesor betweendifferent parts of a single large molecule. ' Help stabilizethe three-dimensional shapeof largemolecules(e.g., DNA and proteins). l. Hydrogen bonds Hydrogen bond : Bond formedby the chargeattractionwhen a hydrogenatom covalentlybondedto one electronegative atom i ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... C) an ionic bond D) a hydrophobic interaction 30) A covalent bond is likely to be polar when _____. A) one of the atoms sharing electrons is more electronegative than the other atom B) the two atoms sharing electrons are equally electronegative C) carbon is one of the two atoms sharing electrons D) ...
... C) an ionic bond D) a hydrophobic interaction 30) A covalent bond is likely to be polar when _____. A) one of the atoms sharing electrons is more electronegative than the other atom B) the two atoms sharing electrons are equally electronegative C) carbon is one of the two atoms sharing electrons D) ...
Polar and Nonpolar Covalent Compounds
... Recall that polarity refers to an unequal sharing of electrons resulting from differences in electronegativity. There is a distinction between polar bonds and polar molecules. A polar covalent bond occurs when bonding electrons are more attracted to an atom with a higher electronegativity. The polar ...
... Recall that polarity refers to an unequal sharing of electrons resulting from differences in electronegativity. There is a distinction between polar bonds and polar molecules. A polar covalent bond occurs when bonding electrons are more attracted to an atom with a higher electronegativity. The polar ...
Chapter 2
... Attractive force between electropositive hydrogen of one molecule and an electronegative atom of another molecule Common between dipoles such as water Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape ...
... Attractive force between electropositive hydrogen of one molecule and an electronegative atom of another molecule Common between dipoles such as water Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape ...
chemical*equations
... When'a'chemical'reaction'occurs,' atoms'rearrange'to'form'new' compounds,'but'no'new'atoms'are' created'nor'are'any'destroyed.'This' concept'is'called'conservation'of' mass.'Mass'conservation'can'be' seen'in'a'balanced'chemical' equation,'where'the'numbers'of' each'kind'of'atom'are'the'same'on' both ...
... When'a'chemical'reaction'occurs,' atoms'rearrange'to'form'new' compounds,'but'no'new'atoms'are' created'nor'are'any'destroyed.'This' concept'is'called'conservation'of' mass.'Mass'conservation'can'be' seen'in'a'balanced'chemical' equation,'where'the'numbers'of' each'kind'of'atom'are'the'same'on' both ...
Part a
... hydrogen of one molecule and an electronegative atom of another molecule ◦ Common between dipoles such as water ◦ Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape ...
... hydrogen of one molecule and an electronegative atom of another molecule ◦ Common between dipoles such as water ◦ Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape ...
I. Why Atoms Combine - Manchester High School
... • formed by transferring efrom a metal to a nonmetal ...
... • formed by transferring efrom a metal to a nonmetal ...
Bond
... A group of atoms held together by covalent bonds is called a molecule. The properties of a molecule, including its role in nature, depends primarily on its molecular structure, or shape. Molecular shape contributes toward determining a compound’s boiling point, freezing point, viscosity, solubility, ...
... A group of atoms held together by covalent bonds is called a molecule. The properties of a molecule, including its role in nature, depends primarily on its molecular structure, or shape. Molecular shape contributes toward determining a compound’s boiling point, freezing point, viscosity, solubility, ...
CHE 0315 SEM 3, 2013/14 TOPIC 5: CHEMICAL BONDING 1. State
... Identify the type of bond described for each of the following as polar covalent, non-polar covalent, or metallic. ...
... Identify the type of bond described for each of the following as polar covalent, non-polar covalent, or metallic. ...
The Chemical Context of Life PPT
... and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more variety consistent with the structural demands required in biological systems. C. Biological conditions are often aqueous, and the water would ...
... and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more variety consistent with the structural demands required in biological systems. C. Biological conditions are often aqueous, and the water would ...
The Chemical Context of Life
... and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more variety consistent with the structural demands required in biological systems. C. Biological conditions are often aqueous, and the water would ...
... and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more variety consistent with the structural demands required in biological systems. C. Biological conditions are often aqueous, and the water would ...
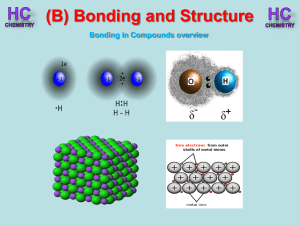
Lesson 1 - Bonding in compounds overview
... Silicon, like carbon, can form giant covalent networks. Silicon carbide exist in a similar structure to diamond. Tetrahedral shape ...
... Silicon, like carbon, can form giant covalent networks. Silicon carbide exist in a similar structure to diamond. Tetrahedral shape ...
Chemistry for BIOS 302
... Chemical Bonds • Atoms can combine with each other to form molecules. Very few elements exist naturally in an uncombined state: mostly they are joined into molecules. • A molecule is a defined number of atoms grouped into a defined spatial relationship. For example, water, H2O, is 2 hydrogen atoms ...
... Chemical Bonds • Atoms can combine with each other to form molecules. Very few elements exist naturally in an uncombined state: mostly they are joined into molecules. • A molecule is a defined number of atoms grouped into a defined spatial relationship. For example, water, H2O, is 2 hydrogen atoms ...
Honors Chemistry
... The additional notes on gases quiz will take place on ___________________________. It will be a 25 point quiz. It will have a 10 point matching section, and multiple short answer sections. You are required to know facts from the packet, equations, and be able to draw Lewis structures of the molecule ...
... The additional notes on gases quiz will take place on ___________________________. It will be a 25 point quiz. It will have a 10 point matching section, and multiple short answer sections. You are required to know facts from the packet, equations, and be able to draw Lewis structures of the molecule ...
nature of Matter
... They form when the electrons of two or more atoms interact. The electrons which are available for bonding are called valence electrons. Depending on how the electrons interact, the type of bond is decided. The main types of chemical bonds are Ionic & Covalent. When electrons are transferred from one ...
... They form when the electrons of two or more atoms interact. The electrons which are available for bonding are called valence electrons. Depending on how the electrons interact, the type of bond is decided. The main types of chemical bonds are Ionic & Covalent. When electrons are transferred from one ...
Elements, mixtures and compounds lecture
... A. exists as only one type of atom: it is, therefore a pure substance (This does not often occur in nature); gold necklace? Oxygen is the most common pure element on Earth (occurs as a dioxide: O2 , what does “di” mean?) B. cannot be broken down by chemical reactions: burning/acids/eating (but nucle ...
... A. exists as only one type of atom: it is, therefore a pure substance (This does not often occur in nature); gold necklace? Oxygen is the most common pure element on Earth (occurs as a dioxide: O2 , what does “di” mean?) B. cannot be broken down by chemical reactions: burning/acids/eating (but nucle ...
2.5 THE NAMES AND FORMULAS OF COMPOUNDS
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
Hydrogen bond
A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (H) atom bound to a highly electronegative atom such as nitrogen (N), oxygen (O) or fluorine (F) experiences attraction to some other nearby highly electronegative atom.These hydrogen-bond attractions can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular). The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have no hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.In 2011, an IUPAC Task Group recommended a modern evidence-based definition of hydrogen bonding, which was published in the IUPAC journal Pure and Applied Chemistry. This definition specifies that The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. An accompanying detailed technical report provides the rationale behind the new definition.