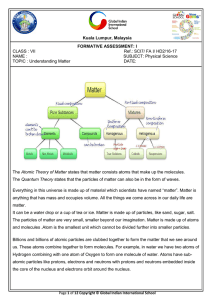
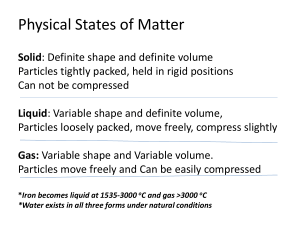
Bio 102 Lecture - chapter 2 The Chemical Basis of Life
... If 3 or less electrons in the outer most shell – Tendency to donate electrons. If 5 or more electrons in the outer most shell – Tendency to receive electrons. A ‘chemical bond’ the force of attraction between atoms to attain stability. ...
... If 3 or less electrons in the outer most shell – Tendency to donate electrons. If 5 or more electrons in the outer most shell – Tendency to receive electrons. A ‘chemical bond’ the force of attraction between atoms to attain stability. ...
worksheer format 11-12
... Plasma is a state of matter in which all matter is ionized and it occurs in the form of ions and electrons. Since, we know that for the ionization of the element or matter to occur energy is required to pull the electron from the attraction of the nuclear charge, high energy is required. So for the ...
... Plasma is a state of matter in which all matter is ionized and it occurs in the form of ions and electrons. Since, we know that for the ionization of the element or matter to occur energy is required to pull the electron from the attraction of the nuclear charge, high energy is required. So for the ...
All That Matters - Teach-n-Learn-Chem
... with other substances. Will it burn? Does it dissolve in water? Does it produce bubbles of gas dropped into acid? All of these things allow us to tell the difference between water and alcohol, for example, and many other substances with the use of only one or two of our senses. List at least five of ...
... with other substances. Will it burn? Does it dissolve in water? Does it produce bubbles of gas dropped into acid? All of these things allow us to tell the difference between water and alcohol, for example, and many other substances with the use of only one or two of our senses. List at least five of ...
CHEM1411,chapter 1-2-3 exercises 1. In 1828, the diameter of the
... 19. Calculate the percent composition by mass of carbon in Na2CO3. 20. Commonly used gases in the laboratory are generally obtained from pressurized metal gas cylinders, but for small amounts of occasionally used gases, it is sometimes easier just to prepare them chemically as needed. For example, n ...
... 19. Calculate the percent composition by mass of carbon in Na2CO3. 20. Commonly used gases in the laboratory are generally obtained from pressurized metal gas cylinders, but for small amounts of occasionally used gases, it is sometimes easier just to prepare them chemically as needed. For example, n ...
APES Lesson 23B (2014-15) - Matter, Chemistry - science-b
... The principle that matter many be transformed for one type of substance into another s, but it cannot be created or destroyed. Autotroph: An organism that produces its own food from inorganic compounds and a source of energy. There are photoautotrophs (photosynthetic plants) and chemical autotrophs. ...
... The principle that matter many be transformed for one type of substance into another s, but it cannot be created or destroyed. Autotroph: An organism that produces its own food from inorganic compounds and a source of energy. There are photoautotrophs (photosynthetic plants) and chemical autotrophs. ...
Chapter 4 REVIEW
... 21. Ionic compounds and metals have different physical properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large differe ...
... 21. Ionic compounds and metals have different physical properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large differe ...
Slide 1
... and after the reaction. Ions such as these, which do not participate directly in a reaction in solution, are called spectator ions. • The ions that participate in this reaction are the Ba2+ and CrO42- ions, which combine to form solid BaCrO4: ...
... and after the reaction. Ions such as these, which do not participate directly in a reaction in solution, are called spectator ions. • The ions that participate in this reaction are the Ba2+ and CrO42- ions, which combine to form solid BaCrO4: ...
Group II Elements - Innovative Education.org
... The Atypical Behaviour of Beryllium. As for any group in the Periodic Table the Group 2 atoms get larger. So do their ions. The ions have a charge of +2 when the atoms lose the two outermost-level electrons, leaving this level empty. The two electrons of the Be2+ ion occupy the first energy level on ...
... The Atypical Behaviour of Beryllium. As for any group in the Periodic Table the Group 2 atoms get larger. So do their ions. The ions have a charge of +2 when the atoms lose the two outermost-level electrons, leaving this level empty. The two electrons of the Be2+ ion occupy the first energy level on ...
The Ecosystem - washburnsciencelies
... mineralizes into soil and some lost thru leeching and gained via weathering of rock. Plants absorb the nutrients and return it to litter through decay. ...
... mineralizes into soil and some lost thru leeching and gained via weathering of rock. Plants absorb the nutrients and return it to litter through decay. ...
Atoms, Ions, and Molecules File
... • Atoms of a given element are all the same. Atoms of different elements are different. • Atoms are not changed into different atoms in a chemical reaction. • Compounds are formed when atoms of two or more elements combine. ...
... • Atoms of a given element are all the same. Atoms of different elements are different. • Atoms are not changed into different atoms in a chemical reaction. • Compounds are formed when atoms of two or more elements combine. ...
lecture 13
... marked the moment we learned to transform brittle iron ores to iron metal. This affected everything from how we grew food to how we waged wars. ...
... marked the moment we learned to transform brittle iron ores to iron metal. This affected everything from how we grew food to how we waged wars. ...
CHEMISTRY-A SCIENCE FOR 21st Century
... *Changes associate with chemical properties result from the interaction of a substance with one or more other substances *Sometimes the presence of energy (heat, light) and pressure also triggers the change. (ex: decomposition of Hydrogen peroxide into water and oxygen) ...
... *Changes associate with chemical properties result from the interaction of a substance with one or more other substances *Sometimes the presence of energy (heat, light) and pressure also triggers the change. (ex: decomposition of Hydrogen peroxide into water and oxygen) ...
Atomic Theories and Models - MrD-Home
... The chemical equation for the reaction of methane and oxygen is ______ yet properly balanced because the atoms of the elements on the product side do not ______ the atoms of each element on the reactant side of the equation. The _________________________, which states that matter can neither be ____ ...
... The chemical equation for the reaction of methane and oxygen is ______ yet properly balanced because the atoms of the elements on the product side do not ______ the atoms of each element on the reactant side of the equation. The _________________________, which states that matter can neither be ____ ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... *f. COMPOUNDS CAN be broken down, but because the elements were CHEMICALLY joined together, a CHEMICAL process is necessary to SEPARATE them *1. Heating breaks down some COMPOUNDS: iron separated from oxygen (e.g.) 2 Fe2O3 + 3 C (are heated) 4 Fe + 3 CO2 (the IRON [Fe] is SEPARATED) *2. Electrolys ...
... *f. COMPOUNDS CAN be broken down, but because the elements were CHEMICALLY joined together, a CHEMICAL process is necessary to SEPARATE them *1. Heating breaks down some COMPOUNDS: iron separated from oxygen (e.g.) 2 Fe2O3 + 3 C (are heated) 4 Fe + 3 CO2 (the IRON [Fe] is SEPARATED) *2. Electrolys ...
Name___________________________________ Physical
... 9) How can you drive the water out of a hydrate? By ________________________. _________ _________ 10) Which of the following correctly shows the formula for a hydrate? A) MgSO4 (H2 O)7 B) H2 O C) H2 O2 ...
... 9) How can you drive the water out of a hydrate? By ________________________. _________ _________ 10) Which of the following correctly shows the formula for a hydrate? A) MgSO4 (H2 O)7 B) H2 O C) H2 O2 ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... *1. Heating breaks down some __________________: iron separated from oxygen (e.g.) 2 Fe2O3 + 3 C (are heated) 4 Fe + 3 CO2 (the _______ [Fe] is SEPARATED) *2. Electrolysis is an ______________ current passed through some COMPOUNDS to break them down: water is broken down into _____________ gas [H2 ...
... *1. Heating breaks down some __________________: iron separated from oxygen (e.g.) 2 Fe2O3 + 3 C (are heated) 4 Fe + 3 CO2 (the _______ [Fe] is SEPARATED) *2. Electrolysis is an ______________ current passed through some COMPOUNDS to break them down: water is broken down into _____________ gas [H2 ...
Haley CHM2045 Final Review
... 2. A 1.0 L mixture of He, Ar, and Ne has a total pressure of 654 mmHg at 298 K. If the partial pressure of He is 378 mmHg and the partial pressure of Ne is 112 mmHg, what is the partial pressure of Ar? 3. Lithium reacts with nitrogen gas in the following reaction, 6Li + N2 —> 2Li3N What mass of lith ...
... 2. A 1.0 L mixture of He, Ar, and Ne has a total pressure of 654 mmHg at 298 K. If the partial pressure of He is 378 mmHg and the partial pressure of Ne is 112 mmHg, what is the partial pressure of Ar? 3. Lithium reacts with nitrogen gas in the following reaction, 6Li + N2 —> 2Li3N What mass of lith ...
Student`s guide - National Centre for Biotechnology Education
... the rate of photosynthesis exceeds the rate of respiration). The colour of hydrogencarbonate indicator can thus be used to monitor both respiration and photosynthesis. The colour change can be measured either by using a colorimeter to measure absorbance at 550 nm (that is, using a green filter) or b ...
... the rate of photosynthesis exceeds the rate of respiration). The colour of hydrogencarbonate indicator can thus be used to monitor both respiration and photosynthesis. The colour change can be measured either by using a colorimeter to measure absorbance at 550 nm (that is, using a green filter) or b ...
urbano, mariajose
... • Are the regions of organic molecules which are commonly chemically reactive. • Behave consistently from one organic molecule to another. • Depending upon their number and arrangement, determine unique chemical properties of organic molecules in which they occur. As with hydrocarbons, diverse organ ...
... • Are the regions of organic molecules which are commonly chemically reactive. • Behave consistently from one organic molecule to another. • Depending upon their number and arrangement, determine unique chemical properties of organic molecules in which they occur. As with hydrocarbons, diverse organ ...
Page 1 of 4 FOSS California Mixtures and Solutions
... Caisson: A large box with no bottom. These boxes were used to provide environments for workers under water. Carbohydrate: A group of carbon-based nutrients, such as sugars and starches. Carbon-14 dating: A process used to find the age of carbon-based matter. Carbon dioxide gas: A compound made from ...
... Caisson: A large box with no bottom. These boxes were used to provide environments for workers under water. Carbohydrate: A group of carbon-based nutrients, such as sugars and starches. Carbon-14 dating: A process used to find the age of carbon-based matter. Carbon dioxide gas: A compound made from ...
Welcome to AP Chemistry!
... I hope you are ready for a fun, yet challenging year. You already have a solid background in basic chemistry from your first year Chem class, and this is critical to success in AP Chem. As the year progresses and you develop your skills for making connections and problem solving as we delve into gre ...
... I hope you are ready for a fun, yet challenging year. You already have a solid background in basic chemistry from your first year Chem class, and this is critical to success in AP Chem. As the year progresses and you develop your skills for making connections and problem solving as we delve into gre ...
Chemistry I Final Review
... 52. How many grams of potassium bromide should be added to water to prepare 0.50 L of solution with a molarity of 0.125 M? ...
... 52. How many grams of potassium bromide should be added to water to prepare 0.50 L of solution with a molarity of 0.125 M? ...
Topic 3&4 Atoms and the per.table
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.