Chemical Reactions
... ___________________. A chemical change may be observed during a chemical reaction when the following happens: (use figure 2.2a-e) 1. ________________________________________________ 2. ________________________________________________ 3. ________________________________________________ 4. ___________ ...
... ___________________. A chemical change may be observed during a chemical reaction when the following happens: (use figure 2.2a-e) 1. ________________________________________________ 2. ________________________________________________ 3. ________________________________________________ 4. ___________ ...
Unit 2
... course. After this page is a sheet of elements; you are expected to know the symbols and names of those elements. The packet is important, but more important is that you understand the material on this. As such you will be tested on these assignments in one big test. The test will occur on the first ...
... course. After this page is a sheet of elements; you are expected to know the symbols and names of those elements. The packet is important, but more important is that you understand the material on this. As such you will be tested on these assignments in one big test. The test will occur on the first ...
Unit 2
... After this page is a sheet of elements; you are expected to know the symbols and names of those elements. The packet is important, but more important is that you understand the material on this. As such you will be tested on these assignments in one big test. The test will occur on the first non-sho ...
... After this page is a sheet of elements; you are expected to know the symbols and names of those elements. The packet is important, but more important is that you understand the material on this. As such you will be tested on these assignments in one big test. The test will occur on the first non-sho ...
- Aboriginal Access to Engineering
... properties in the final product like strength, chemical-resistance or hardness. For example, the addition of chromium in carefully measured amounts creates stainless steel, something you probably have in your home. Stainless steel is used to make cutlery, sinks and surgical tools because of its high ...
... properties in the final product like strength, chemical-resistance or hardness. For example, the addition of chromium in carefully measured amounts creates stainless steel, something you probably have in your home. Stainless steel is used to make cutlery, sinks and surgical tools because of its high ...
amino acids
... through atmospheric CO2 (through what processes?) Photosynthesis and Respiration ...
... through atmospheric CO2 (through what processes?) Photosynthesis and Respiration ...
Physics 1010: The Physics of Everyday Life
... b) The thin layer helps focus microwaves into the surface of the ...
... b) The thin layer helps focus microwaves into the surface of the ...
Chemical Reactions
... How do you get Fe2 from Fe and O3 from O2? We do this by balancing equations. 4Fe + 3O2 → 2Fe2O3 You cannot make or destroy matter! It also helps to know if it is solid, liquid or gas. 4Fe(s) + 3O2(g)→ 2Fe2O3(s) ...
... How do you get Fe2 from Fe and O3 from O2? We do this by balancing equations. 4Fe + 3O2 → 2Fe2O3 You cannot make or destroy matter! It also helps to know if it is solid, liquid or gas. 4Fe(s) + 3O2(g)→ 2Fe2O3(s) ...
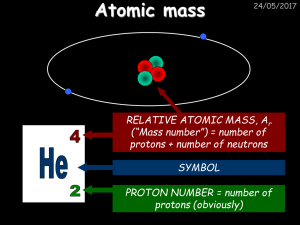
Atomic mass - drseemaljelani
... Ammonia is a very important chemical as it can be used to make plant fertilisers and nitric acid: Ammonia gas ...
... Ammonia is a very important chemical as it can be used to make plant fertilisers and nitric acid: Ammonia gas ...
Experiment 11 – Comparison of the Energy Content of Fuels
... fuels. The fuels will be divided into two classes – hydrocarbons and alcohols. We’ll try to discover how energy content changes when we go from hydrocarbons to alcohols, and how it changes as the size of the fuel molecule changes. Why are we interested in this? We are interested because of the impor ...
... fuels. The fuels will be divided into two classes – hydrocarbons and alcohols. We’ll try to discover how energy content changes when we go from hydrocarbons to alcohols, and how it changes as the size of the fuel molecule changes. Why are we interested in this? We are interested because of the impor ...
CHEMISTRY I Final..#1..rev 4KEY
... Objective 2.07: Assess covalent bonding in molecular compounds as related to chemical and physical properties and molecular geometry. 38. The boiling point of HBr is lower than that of HF because: a. HBr is heavier than HF and therefore it requires less energy to vaporize. b. HBr has dipole-dipole ...
... Objective 2.07: Assess covalent bonding in molecular compounds as related to chemical and physical properties and molecular geometry. 38. The boiling point of HBr is lower than that of HF because: a. HBr is heavier than HF and therefore it requires less energy to vaporize. b. HBr has dipole-dipole ...
Chemistry2 Midterm Review 2012 – Tuesday
... Include a labeled y-axis, reactants, products, activated complex, and change in enthalpy. 54. What is the difference between a state function and a path function? Provide an example of each. 55. Consider the following reaction: CH3OH(g) CO(g) + 2H2(g) ΔH= +90.7 kJ a. Is the reaction endothermic or ...
... Include a labeled y-axis, reactants, products, activated complex, and change in enthalpy. 54. What is the difference between a state function and a path function? Provide an example of each. 55. Consider the following reaction: CH3OH(g) CO(g) + 2H2(g) ΔH= +90.7 kJ a. Is the reaction endothermic or ...
CAMBRIDGE INTERNATIONAL EXAMINATIONS
... There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C, and D. Choose the one you consider correct and record your choice in soft pencil on the separate answer sheet. Read the instructions on the Answer Sheet very carefully. Each corr ...
... There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C, and D. Choose the one you consider correct and record your choice in soft pencil on the separate answer sheet. Read the instructions on the Answer Sheet very carefully. Each corr ...
Glossary (PDF file)
... acidic. Stomach acid has a pH of about 2. A high pH means a solution is basic. Ammonia solution used for cleaning is a base and has a pH of about 11. polymer A large molecule made from chemical reactions between many small molecules. Poly means “many.” When many monomers react with each other, they ...
... acidic. Stomach acid has a pH of about 2. A high pH means a solution is basic. Ammonia solution used for cleaning is a base and has a pH of about 11. polymer A large molecule made from chemical reactions between many small molecules. Poly means “many.” When many monomers react with each other, they ...
Chemical Reactions: Introduction to Reaction Types
... elements, b) 1 element and 1 binary compound (consisting of 2 elements), or c) 2 binary compounds. The following are examples of combination reactions: The rusting of iron: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) The formation of one kind of acid rain: SO3 (g) + H2O (l) → H2SO4 (aq) 2. Decomposition: AB → A ...
... elements, b) 1 element and 1 binary compound (consisting of 2 elements), or c) 2 binary compounds. The following are examples of combination reactions: The rusting of iron: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) The formation of one kind of acid rain: SO3 (g) + H2O (l) → H2SO4 (aq) 2. Decomposition: AB → A ...
Precipitate Lab Report Power Point with Answers
... Temperature change, odor change, precipitate formation, irreversibility, color change, and new bubble formation are the evidence for a chemical reaction occuring. Not every time one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no o ...
... Temperature change, odor change, precipitate formation, irreversibility, color change, and new bubble formation are the evidence for a chemical reaction occuring. Not every time one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no o ...
Chapter 5
... enzymatically catalyzed chemical reactions in a cell Metabolic pathways are determined by enzymes Enzymes are encoded by genes ...
... enzymatically catalyzed chemical reactions in a cell Metabolic pathways are determined by enzymes Enzymes are encoded by genes ...
8F Compounds and Mixtures
... substances that are mixed together but have not reacted with each other. Sea water is a mixture of salts, water and other substances. A mixture is not the same as a compound: 1. The proportions of the substances in a mixture are not fixed. 2. The properties of a mixture are often an “average” of the ...
... substances that are mixed together but have not reacted with each other. Sea water is a mixture of salts, water and other substances. A mixture is not the same as a compound: 1. The proportions of the substances in a mixture are not fixed. 2. The properties of a mixture are often an “average” of the ...
CHEM 13 NEWS EXAM 1998 - University of Waterloo
... Fe loses electrons less readily than Mg, making Mg the anode. ...
... Fe loses electrons less readily than Mg, making Mg the anode. ...
Unit 3 Practice Test
... 12. The group of elements that forms oxides with the general formula XO is A. Group 1 (IA) ...
... 12. The group of elements that forms oxides with the general formula XO is A. Group 1 (IA) ...
Unit 2
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
Unit 2
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
Units 3 and 4 Revision
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
Biochemistry I (CHE 418 / 5418)
... • Combustion – special type of oxidation / reduction reaction involving oxygen. CH4(g) + 2O2 → CO2(g) + 2H2O • Organic Chemist identify oxidation / reduction – An atom is oxidized if: • Gains electrons OR – Attached to more oxygen in product than reactant ...
... • Combustion – special type of oxidation / reduction reaction involving oxygen. CH4(g) + 2O2 → CO2(g) + 2H2O • Organic Chemist identify oxidation / reduction – An atom is oxidized if: • Gains electrons OR – Attached to more oxygen in product than reactant ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.