09 Stoichiometry WS Stoichiometry WS
... b. If 0.905 mol Al2O3 is produced in the reaction, what mass of Fe is produced? c. How many moles of Fe2O3 will react with 99.0 g of Al? 3. The reaction N2(g) + 3H2(g) 2NH3(g) is used to produce ammonia commercially. If 1.40g of N2 are used in the reaction, how many grams of H2 will be needed? 4. Wh ...
... b. If 0.905 mol Al2O3 is produced in the reaction, what mass of Fe is produced? c. How many moles of Fe2O3 will react with 99.0 g of Al? 3. The reaction N2(g) + 3H2(g) 2NH3(g) is used to produce ammonia commercially. If 1.40g of N2 are used in the reaction, how many grams of H2 will be needed? 4. Wh ...
makeup2
... freezes at -1.46°C. What is the molecular weight of x? (Kf = 1.86) (A) 84 x 1.86 x 1.46 = 222 g/mol (B) 84 x (1.86 / 1.46) = 107 g/mol (C) 84 x (1.46 / 1.86) = 66 g/mol (D) 1.46 x (1.86 / 84) = 0.032 g/mol 59. A 1.20 liter sample is drawn from a bottle labeled "86.0% by weight H2SO4, density 1.787 g ...
... freezes at -1.46°C. What is the molecular weight of x? (Kf = 1.86) (A) 84 x 1.86 x 1.46 = 222 g/mol (B) 84 x (1.86 / 1.46) = 107 g/mol (C) 84 x (1.46 / 1.86) = 66 g/mol (D) 1.46 x (1.86 / 84) = 0.032 g/mol 59. A 1.20 liter sample is drawn from a bottle labeled "86.0% by weight H2SO4, density 1.787 g ...
quant6stoichiom
... ex. out in space Carbon dioxide that is produced by astronauts can be removed with lithium hydroxide. The reaction produces lithium carbonate and water. An astronaut produces an average of 1.00 x 103 g carbon dioxide per day. How much lithium hydroxide should engineers put on the spaceship per astro ...
... ex. out in space Carbon dioxide that is produced by astronauts can be removed with lithium hydroxide. The reaction produces lithium carbonate and water. An astronaut produces an average of 1.00 x 103 g carbon dioxide per day. How much lithium hydroxide should engineers put on the spaceship per astro ...
Chemistry 1st Semester Practice Exam
... expect to be ionic? A. H2O B. CO2 51. Which group of elements is most likely to form ions by losing one electron? ...
... expect to be ionic? A. H2O B. CO2 51. Which group of elements is most likely to form ions by losing one electron? ...
A.P. Chemistry
... M = mol/L Volume in liters (be sure to convert mL L) (p. 145-147) Problem: What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution? ...
... M = mol/L Volume in liters (be sure to convert mL L) (p. 145-147) Problem: What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H2SO4 solution? ...
Chapter 4 - U of L Class Index
... ___ mole of CH4 reacts with ___ moles of O2 to make ___ mole of CO2 and ___ moles of H2O If we burn 100 grams of CH4 with enough oxygen, what mass of water is produced (assuming complete reaction)? CH4 + 2 O2 ...
... ___ mole of CH4 reacts with ___ moles of O2 to make ___ mole of CO2 and ___ moles of H2O If we burn 100 grams of CH4 with enough oxygen, what mass of water is produced (assuming complete reaction)? CH4 + 2 O2 ...
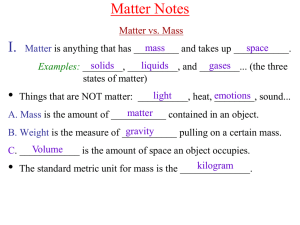
Chem A Week 2 Matter Notes
... of superheated “soup” of bits and pieces of atoms. It only exists ...
... of superheated “soup” of bits and pieces of atoms. It only exists ...
4 - Ms McRae`s Science
... a)yes bec an increase in the temperature of the HCl will increase the velocity of the reactant particles which will increase the number of collisions AND increase the number of effective collisions i.e. ones that have sufficient energy (activation energy) to react b) skipping this one for now until ...
... a)yes bec an increase in the temperature of the HCl will increase the velocity of the reactant particles which will increase the number of collisions AND increase the number of effective collisions i.e. ones that have sufficient energy (activation energy) to react b) skipping this one for now until ...
Toluenediamine
... The reduction of dinitrotoluene is characterized by its strong exotherm of >1100 kJ/mol. Due to this fact combined with the thermal instability of the dinitro compound certain processing requirements must be considered. Gas-phase reaction of dinitrotoluene can merely be realized and particular preca ...
... The reduction of dinitrotoluene is characterized by its strong exotherm of >1100 kJ/mol. Due to this fact combined with the thermal instability of the dinitro compound certain processing requirements must be considered. Gas-phase reaction of dinitrotoluene can merely be realized and particular preca ...
are physical changes - Chemistry Information Site
... substances using physical or chemical means - Elements are the building blocks of chemistry! They are the simple things from which all other things are formed! ...
... substances using physical or chemical means - Elements are the building blocks of chemistry! They are the simple things from which all other things are formed! ...
Inorganic Chemistry Lesson 3
... formed by silver and oxygen has a formula Ag2 O. Using this information, can you predict a formula of a compound containing silver and chlorine? It is intuitively clear that, since oxygen binds to two atoms of hydrogen, valence of oxygen is as twice as big as valence of hydrogen. H2 O and Ag2 O form ...
... formed by silver and oxygen has a formula Ag2 O. Using this information, can you predict a formula of a compound containing silver and chlorine? It is intuitively clear that, since oxygen binds to two atoms of hydrogen, valence of oxygen is as twice as big as valence of hydrogen. H2 O and Ag2 O form ...
Exam #2
... respectively, which organism could get more energy for growth, organism A that oxidizes methane and reduces sulfate, or organism B that oxidizes hydrogen sulfide and reduces iron? Justify your answer in terms of volts. It may be useful to diagram the electron transfer between couples for each. Organ ...
... respectively, which organism could get more energy for growth, organism A that oxidizes methane and reduces sulfate, or organism B that oxidizes hydrogen sulfide and reduces iron? Justify your answer in terms of volts. It may be useful to diagram the electron transfer between couples for each. Organ ...
Covalent Bonds - WordPress.com
... • A molecule’s shape is usually very important to its function • A molecule’s shape is determined by the positions of its atoms’ valence orbitals • Biological molecules recognize and interact with each other with a specificity based on ...
... • A molecule’s shape is usually very important to its function • A molecule’s shape is determined by the positions of its atoms’ valence orbitals • Biological molecules recognize and interact with each other with a specificity based on ...
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... 10. In “2s2 “, the 2 represents which 3 Æ (column, group, family, period, row or energy level). 7 Ions and Bonding 11. Which 2 have 3 valence electron? ...
... 10. In “2s2 “, the 2 represents which 3 Æ (column, group, family, period, row or energy level). 7 Ions and Bonding 11. Which 2 have 3 valence electron? ...
Topic 1 Assignment File
... When 3.3 moles of Ni react with 159.3 grams of AgNO3, which is the limiting and which is the excess ...
... When 3.3 moles of Ni react with 159.3 grams of AgNO3, which is the limiting and which is the excess ...
Electrochemistry Lecture
... Oxidizing agent; That which is responsible to oxidize another. O2 ; Oxidizing agent; The agent itself undergoes reduction ...
... Oxidizing agent; That which is responsible to oxidize another. O2 ; Oxidizing agent; The agent itself undergoes reduction ...
Redox - edl.io
... Exercise 17 For this reaction, identify the atoms that are oxidized and reduced, and specify the oxidizing and reducing ...
... Exercise 17 For this reaction, identify the atoms that are oxidized and reduced, and specify the oxidizing and reducing ...
half-reactions - Clayton State University
... Nonspontaneous Process - Requires something to be applied in order for it to occur (usually in the form of energy) ...
... Nonspontaneous Process - Requires something to be applied in order for it to occur (usually in the form of energy) ...
The Atomic Theory
... The formula gave excellent agreement with the experimental results. However, the concept of discrete energy states or quanta was so different from classical theory that it was met with strong opposition. Hertz (1887) had discovered that certain metals emit electrons when irradiated with UV light. In ...
... The formula gave excellent agreement with the experimental results. However, the concept of discrete energy states or quanta was so different from classical theory that it was met with strong opposition. Hertz (1887) had discovered that certain metals emit electrons when irradiated with UV light. In ...
S.O.L. Review
... B. It has the same number of protons and two more electrons than C-12 C. It has the same number of protons but two more neutrons than C-12 D. It has a different number of protons and two more neutrons than C-12 ...
... B. It has the same number of protons and two more electrons than C-12 C. It has the same number of protons but two more neutrons than C-12 D. It has a different number of protons and two more neutrons than C-12 ...
Chemistry for BIOS 302
... • Atoms can combine with each other to form molecules. Very few elements exist naturally in an uncombined state: mostly they are joined into molecules. • A molecule is a defined number of atoms grouped into a defined spatial relationship. For example, water, H2O, is 2 hydrogen atoms connected to an ...
... • Atoms can combine with each other to form molecules. Very few elements exist naturally in an uncombined state: mostly they are joined into molecules. • A molecule is a defined number of atoms grouped into a defined spatial relationship. For example, water, H2O, is 2 hydrogen atoms connected to an ...
2002 Final Exam for Practice - Department of Chemistry | Oregon
... Fill in the front page of the Scantron answer sheet with your last name, first name, middle initial, and student identification number. Leave the class section number and the test form number blank. This exam consists of 27 multiple-choice questions and 5 open-ended questions. Each question has five ...
... Fill in the front page of the Scantron answer sheet with your last name, first name, middle initial, and student identification number. Leave the class section number and the test form number blank. This exam consists of 27 multiple-choice questions and 5 open-ended questions. Each question has five ...
primes - The Institute of Mathematical Sciences
... Even if we know whether a number is prime or composite, finding a factor might still be very difficult. In 1876 mathematicians discovered that the number 267-1 (this is very long to write down so I will not even try) is composite, but they did not know any of its factors. Of course, 267 has only one ...
... Even if we know whether a number is prime or composite, finding a factor might still be very difficult. In 1876 mathematicians discovered that the number 267-1 (this is very long to write down so I will not even try) is composite, but they did not know any of its factors. Of course, 267 has only one ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.