Environment: The Science Behind the Stories, 4e (Withgott)
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
FREE Sample Here
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
metal-water interactions and hydrogen bond strength
... (water molecules as hydrogen bond donors and acceptors) [1,2,5 and Refs. therein]; (iii) anti-cooperative or competitive effect (different proton acceptor strength of the atoms building up one acceptor group) [1-3 and Refs. therein]; (iv) repulsive potential forces at the respective lattice sites (i ...
... (water molecules as hydrogen bond donors and acceptors) [1,2,5 and Refs. therein]; (iii) anti-cooperative or competitive effect (different proton acceptor strength of the atoms building up one acceptor group) [1-3 and Refs. therein]; (iv) repulsive potential forces at the respective lattice sites (i ...
2. Essential Chemistry
... o Participates in chemical reactions o Water has a high specific heat which moderates temperature - absorbs and releases heat very slowly, minimizes temperature fluctuations to within limits that permit life Heat is absorbed when hydrogen bonds break Heat is released when hydrogen bonds form o R ...
... o Participates in chemical reactions o Water has a high specific heat which moderates temperature - absorbs and releases heat very slowly, minimizes temperature fluctuations to within limits that permit life Heat is absorbed when hydrogen bonds break Heat is released when hydrogen bonds form o R ...
H 2 O
... matter are called chemical reactions • Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells – These interactions usually result in atoms staying close together – The atoms are held together by chemical bonds ...
... matter are called chemical reactions • Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells – These interactions usually result in atoms staying close together – The atoms are held together by chemical bonds ...
Unit 2 Test Review - Liberty High School
... Matter and Atomic Structure Complete the following problems to help prepare you for you Unit 2 Test. You more than likely will want to answer these questions on a separate piece of paper (unless you can write microscopically). In addition to these problems, review your notes, assignments (#29-44), l ...
... Matter and Atomic Structure Complete the following problems to help prepare you for you Unit 2 Test. You more than likely will want to answer these questions on a separate piece of paper (unless you can write microscopically). In addition to these problems, review your notes, assignments (#29-44), l ...
Chapter 11 Chemical Reactions
... equation (You can only change coefficients) – If you change the subscript (formula) you are describing a different chemical. – H2O is a different compound than H2O2 Never put a coefficient in the middle of a formula; they must go only in the front ...
... equation (You can only change coefficients) – If you change the subscript (formula) you are describing a different chemical. – H2O is a different compound than H2O2 Never put a coefficient in the middle of a formula; they must go only in the front ...
Task - Science - Grade 6 - Chemical Reactions
... Explosive reactions are influenced by several factors. One of those factors is the surface area of the substance. When a substance is finely divided, it will normally produce a faster reaction than if the same mass is present as a single lump. A sugar cube has a specific surface area, but, when it i ...
... Explosive reactions are influenced by several factors. One of those factors is the surface area of the substance. When a substance is finely divided, it will normally produce a faster reaction than if the same mass is present as a single lump. A sugar cube has a specific surface area, but, when it i ...
C14_-_Organic_Chemistry
... STUDENT CHECKLIST C14 – Organic Chemistry At the end of this topic, you should: CORE ...
... STUDENT CHECKLIST C14 – Organic Chemistry At the end of this topic, you should: CORE ...
Chapter 18 Review 18.1 Oxidation-Reduction Reactions Oxidation
... - batteries uses energy from an redox reaction to produce an electric current - Electrochemistry involves two types or processes: 1. the production of an electric current from a redox reaction 2. the use of an electric current to produce chemical change - to use energy from the reaction, the oxidizi ...
... - batteries uses energy from an redox reaction to produce an electric current - Electrochemistry involves two types or processes: 1. the production of an electric current from a redox reaction 2. the use of an electric current to produce chemical change - to use energy from the reaction, the oxidizi ...
CH03_Tro_LectureNotes - Tutor
... How do we differentiate a sample of matter from another? When we compare samples of matter, we look at the properties or characteristics that distinguish them from one another. The properties may be chemical properties or physical properties. Chemical properties describe the composition (what is it ...
... How do we differentiate a sample of matter from another? When we compare samples of matter, we look at the properties or characteristics that distinguish them from one another. The properties may be chemical properties or physical properties. Chemical properties describe the composition (what is it ...
Lab 5 - Photosynthesis - philipdarrenjones.com
... 1. Obtain three (3) clean 10-ml syringes (with the same dimensions) and check to make certain that they can create and maintain a vacuum (This procedure will be demonstrated in lab.). The syringes will act as miniature photosynthesis chambers. The following directions will be described for a single ...
... 1. Obtain three (3) clean 10-ml syringes (with the same dimensions) and check to make certain that they can create and maintain a vacuum (This procedure will be demonstrated in lab.). The syringes will act as miniature photosynthesis chambers. The following directions will be described for a single ...
word-doc Practice for the final exam!
... d. pure substance e. solid 3. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is ________. a. a heterogeneous mixture b. an element c. a homogeneous mixture d. a compound e. a mixt ...
... d. pure substance e. solid 3. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is ________. a. a heterogeneous mixture b. an element c. a homogeneous mixture d. a compound e. a mixt ...
eastern illinois university
... 28. Given the reaction: Al2O3 (s) + 6HCl (aq) 2AlCl3 (aq) + 3H2O (l). How many moles of HCl are required to completely react with 0.25 moles of Al2O3? a. 0.042 b. 0.25 c. 0.75 d. 1.5 e. 4 29. The final reaction in the manufacture of nitric acid, HNO3, is: 3NO2 (g) + H2O (l) 2HNO3 (aq) + NO (g). ...
... 28. Given the reaction: Al2O3 (s) + 6HCl (aq) 2AlCl3 (aq) + 3H2O (l). How many moles of HCl are required to completely react with 0.25 moles of Al2O3? a. 0.042 b. 0.25 c. 0.75 d. 1.5 e. 4 29. The final reaction in the manufacture of nitric acid, HNO3, is: 3NO2 (g) + H2O (l) 2HNO3 (aq) + NO (g). ...
Presentation - Chem Rxns - stpats-sch3u-sem1-2013
... Metallic carbonates, when heated, form metallic oxides and CO2(g). EX. CaCO3(s) → CaO(s) + CO2(g) Most metallic hydroxides, when heated, decompose into metallic oxides and water. EX. Ca(OH)2(s) → CaO(s) + H2O(g) Metallic chlorates, when heated, decompose into metallic chlorides and oxygen. EX. 2KClO ...
... Metallic carbonates, when heated, form metallic oxides and CO2(g). EX. CaCO3(s) → CaO(s) + CO2(g) Most metallic hydroxides, when heated, decompose into metallic oxides and water. EX. Ca(OH)2(s) → CaO(s) + H2O(g) Metallic chlorates, when heated, decompose into metallic chlorides and oxygen. EX. 2KClO ...
IOSR Journal of Electrical and Electronics Engineering (IOSR-JEEE)
... energy to induce vectorial electron transfer reaction. Thus DSSCs have the following advantages comparing with the Si based photovoltaic: (1) It is not sensitive to the defects in semiconductors such as defects in Si. (2) The semiconductor-electrolyte Interface (SEI) is easy to form and it is cost e ...
... energy to induce vectorial electron transfer reaction. Thus DSSCs have the following advantages comparing with the Si based photovoltaic: (1) It is not sensitive to the defects in semiconductors such as defects in Si. (2) The semiconductor-electrolyte Interface (SEI) is easy to form and it is cost e ...
Balancing Equations
... Remember, each element has its own symbol(s) - all found in the periodic table ...
... Remember, each element has its own symbol(s) - all found in the periodic table ...
Equation Chapter 1 Section 1 Tips for Studying: Take responsibility
... Mechanical Energy Total mechanical energy of an object in motion is potential and kinetic energy combined. Mechanical energy = Joules = kg . m/s2 . m Mechanical energy = Potential energy + Kinetic energy ...
... Mechanical Energy Total mechanical energy of an object in motion is potential and kinetic energy combined. Mechanical energy = Joules = kg . m/s2 . m Mechanical energy = Potential energy + Kinetic energy ...
AP Chemistry
... I have taught AP Chemistry for 10 years and am very excited about next year. AP Chemistry is designed to prepare you to be successful in college chemistry as well as to pass the AP Chemistry test. Attached is the summer work placket to prepare you. Expect a test on this material the second day of sc ...
... I have taught AP Chemistry for 10 years and am very excited about next year. AP Chemistry is designed to prepare you to be successful in college chemistry as well as to pass the AP Chemistry test. Attached is the summer work placket to prepare you. Expect a test on this material the second day of sc ...
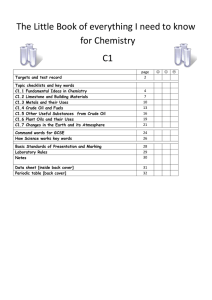
The Big book of C1 chemistry
... Solid particles may contain soot (carbon) and unburnt fuels. The combustion of hydrocarbon fuels releases energy. During combustion the carbon and hydrogen in the fuels are oxidised. Hydrogen is always completely oxidised to water. In complete combustion, the carbon is fully oxidised to carbon d ...
... Solid particles may contain soot (carbon) and unburnt fuels. The combustion of hydrocarbon fuels releases energy. During combustion the carbon and hydrogen in the fuels are oxidised. Hydrogen is always completely oxidised to water. In complete combustion, the carbon is fully oxidised to carbon d ...
Medical Physics and Statistics
... The student surveyed 50 people for support of the proposal: Forty three (43) signed immediately. Six (6) asked for time to think. ...
... The student surveyed 50 people for support of the proposal: Forty three (43) signed immediately. Six (6) asked for time to think. ...
PPT Oxidation
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
PPT Oxidation
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
... There are three other chemical species available in a basic solution besides the ones shown above. They are: ...
Chemical Reactions - TSHSChemistry
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... *f. COMPOUNDS can be broken down, but because the elements were CHEMICALLY joined together, a CHEMICAL process is necessary to SEPARATE them. *1. Heating breaks down some COMPOUNDS: iron separated from oxygen (e.g.) 2 Fe2O3 + 3 C (are heated) 4 Fe + 3 CO2 (the IRON [Fe] is SEPARATED) *2. Electroly ...
... *f. COMPOUNDS can be broken down, but because the elements were CHEMICALLY joined together, a CHEMICAL process is necessary to SEPARATE them. *1. Heating breaks down some COMPOUNDS: iron separated from oxygen (e.g.) 2 Fe2O3 + 3 C (are heated) 4 Fe + 3 CO2 (the IRON [Fe] is SEPARATED) *2. Electroly ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.