CHEMISTRY PHYSICAL SETTING Thursday, PS/CHEMISTRY
... Base your answers to questions 59 through 61 on the information below. Carbon forms molecular compounds with some elements from Group 16. Two of these compounds are carbon dioxide, CO2, and carbon disulfide, CS2. Carbon dioxide is a colorless, odorless gas at room temperature. At standard temperatur ...
... Base your answers to questions 59 through 61 on the information below. Carbon forms molecular compounds with some elements from Group 16. Two of these compounds are carbon dioxide, CO2, and carbon disulfide, CS2. Carbon dioxide is a colorless, odorless gas at room temperature. At standard temperatur ...
the nakuru district sec. schools trial examinations - 2015
... The reaction is exothermic ½ producing heat which decomposes carbon(IV)oxide to black½ carbon specks and oxygen gas. Oxygen gas supports½ continued burning of magnesium to produce white½ magnesium oxide. (b) Write an equation for the reaction in (a) above ...
... The reaction is exothermic ½ producing heat which decomposes carbon(IV)oxide to black½ carbon specks and oxygen gas. Oxygen gas supports½ continued burning of magnesium to produce white½ magnesium oxide. (b) Write an equation for the reaction in (a) above ...
Effects of antioxidants for the degradation of flame
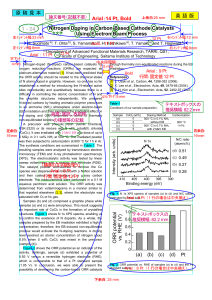
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
Chemistry I Exams and Answer Keys 2015 Season
... As we proceed from left to right in period 3 of the Periodic Table of the elements, we note a decrease in the atomic radius. Which statement correctly explains this phenomenon? A. The number of valence electrons increases, causing an increased attraction between the nucleus and valence electrons. B. ...
... As we proceed from left to right in period 3 of the Periodic Table of the elements, we note a decrease in the atomic radius. Which statement correctly explains this phenomenon? A. The number of valence electrons increases, causing an increased attraction between the nucleus and valence electrons. B. ...
Power point types of chemical rxn
... http://www.youtube.com/watch?v=_P5hGzA6Vb0 See page 263 (c) McGraw Hill Ryerson 2007 ...
... http://www.youtube.com/watch?v=_P5hGzA6Vb0 See page 263 (c) McGraw Hill Ryerson 2007 ...
CHEM%1212K% Final%Exam% Summer%2011% K
... 7.%%At%425oC,%K%=%4.18%x%10J9%for%the%reaction%2HBr(g)% %H2(g)%+%Br2(g).%%If%0.20%bar%of% HBr(g),%and%0.010%bar%of%both%H2(g)%and%Br2(g)%are%introduced%into%a%container,%then% which%expression%best%represents%the%equilibrium%pressure%of%HBr?% ...
... 7.%%At%425oC,%K%=%4.18%x%10J9%for%the%reaction%2HBr(g)% %H2(g)%+%Br2(g).%%If%0.20%bar%of% HBr(g),%and%0.010%bar%of%both%H2(g)%and%Br2(g)%are%introduced%into%a%container,%then% which%expression%best%represents%the%equilibrium%pressure%of%HBr?% ...
Section 1-2 Matter and Its Properties
... An atom is the smallest unit of an element that maintains the properties of that element. An element is a pure substance made up of only one kind of atom. A compound is a substance that is made from the atoms of two or more elements that ...
... An atom is the smallest unit of an element that maintains the properties of that element. An element is a pure substance made up of only one kind of atom. A compound is a substance that is made from the atoms of two or more elements that ...
AP Chemistry Summer Assignment
... Welcome to AP Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. Wi ...
... Welcome to AP Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. Wi ...
AP Chemistry Summer Assignment
... Welcome to AP Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. Wi ...
... Welcome to AP Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. Wi ...
AP Chemistry Summer Assignment
... Welcome to AP Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. Wi ...
... Welcome to AP Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. Wi ...
organic chemistry - Peoria Public Schools
... amount of bromine water which needs to be added before it retains its colour means the greater the degree of unsaturation; the greater the number of double bonds. ...
... amount of bromine water which needs to be added before it retains its colour means the greater the degree of unsaturation; the greater the number of double bonds. ...
- Deans Community High School
... ii) which is the better catalyst for the reaction? Explain your choice. d) The gold and platinum catalysts are used in the solid state. Are the catalysts heterogeneous or homogeneous catalysts? Explain your choice. 32. An advice leaflet given to motorists when catalytic converters were first used st ...
... ii) which is the better catalyst for the reaction? Explain your choice. d) The gold and platinum catalysts are used in the solid state. Are the catalysts heterogeneous or homogeneous catalysts? Explain your choice. 32. An advice leaflet given to motorists when catalytic converters were first used st ...
1.0 basic concepts
... Ag1+(aq) and NO31-(aq) are dissociated ions As they are different, they are not spectator ions and cannot be cancelled out 6) Rewrite the balanced ionic equation without the spectator ions ...
... Ag1+(aq) and NO31-(aq) are dissociated ions As they are different, they are not spectator ions and cannot be cancelled out 6) Rewrite the balanced ionic equation without the spectator ions ...
Writing Formulas Worksheet 1. sodium nitrate 16. aluminum sulfide
... UNIT 7 - CHEMICAL FORMULAS EMPIRICAL FORMULA OF A HYDRATE LAB Many salts crystallized from water solutions appear to be perfectly dry, yet when heated, they liberate large quantities of water. The crystals change form, even color, as the water is driven off. Such compounds are called hydrates. The ...
... UNIT 7 - CHEMICAL FORMULAS EMPIRICAL FORMULA OF A HYDRATE LAB Many salts crystallized from water solutions appear to be perfectly dry, yet when heated, they liberate large quantities of water. The crystals change form, even color, as the water is driven off. Such compounds are called hydrates. The ...
Rxn Pred students
... non-spontaneous redox reaction is brought about by the passage of current under sufficient external electrical potential. The devices in which electrolysis reactions occur are called electrolytic cells. ...
... non-spontaneous redox reaction is brought about by the passage of current under sufficient external electrical potential. The devices in which electrolysis reactions occur are called electrolytic cells. ...
Campbell Biology in Focus (Urry) Chapter 2 The Chemical Context
... 11) An atom has 6 electrons in its outer shell. How many unpaired electrons does it have? A) 0 B) 2 C) 4 D) 6 E) 2 or 4 12) The atomic number of nitrogen is 7. Nitrogen-15 is heavier than nitrogen-14 because the atomic nucleus of nitrogen-15 contains how many neutrons? A) 6 B) 7 C) 8 D) 12 E) 14 13 ...
... 11) An atom has 6 electrons in its outer shell. How many unpaired electrons does it have? A) 0 B) 2 C) 4 D) 6 E) 2 or 4 12) The atomic number of nitrogen is 7. Nitrogen-15 is heavier than nitrogen-14 because the atomic nucleus of nitrogen-15 contains how many neutrons? A) 6 B) 7 C) 8 D) 12 E) 14 13 ...
Chemical Reactions Chemistry - is the study of matter, its properties
... hydrogen atoms, and secondly it could contain other elements bonded to either of the above mentioned elements. Inorganic compounds are any compound which does not involve any form of hydrocarbons. Inorganic compounds are molecular compounds which may contain carbon, but it will be bonded to an eleme ...
... hydrogen atoms, and secondly it could contain other elements bonded to either of the above mentioned elements. Inorganic compounds are any compound which does not involve any form of hydrocarbons. Inorganic compounds are molecular compounds which may contain carbon, but it will be bonded to an eleme ...
File
... his or her arm destroyed by acid, the make-up artist places a thin layer of baking soda in the make-up. When the acid (a mild solution of vinegar) is sprayed, a chemical reaction takes place. The actor’s skin appears to melt away. The foaming skin is really carbon dioxide gas and make-up rolling off ...
... his or her arm destroyed by acid, the make-up artist places a thin layer of baking soda in the make-up. When the acid (a mild solution of vinegar) is sprayed, a chemical reaction takes place. The actor’s skin appears to melt away. The foaming skin is really carbon dioxide gas and make-up rolling off ...
AP Chemistry Summer Assignment
... Welcome to AP/IB Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. ...
... Welcome to AP/IB Chemistry. I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. ...
Chem 1 Worksheets WSHEET 1: Working with Numbers Practice
... 1. Calcium fluoride, CaF2, is a source of fluorine and is used to fluoridate drinking water. Calculate its molar mass. 2. Calculate the molar mass of Ca(BO2)2·6H2O. 3. Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide, Mg(OH)2. 4. Calculate the number of oxygen atoms in 29.3 ...
... 1. Calcium fluoride, CaF2, is a source of fluorine and is used to fluoridate drinking water. Calculate its molar mass. 2. Calculate the molar mass of Ca(BO2)2·6H2O. 3. Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide, Mg(OH)2. 4. Calculate the number of oxygen atoms in 29.3 ...
REVIEW OF HYDROGEN CONVERSION TECHNOLOGIES Abstract: F. Barbir Clean Energy Research Institute
... Hydrogen and electricity are often considered as complementary energy carriers for the future. Hydrogen has some unique properties, which in conjunction with electricity make it an ideal energy carrier or fuel [1,2]: • Just as electricity hydrogen can be produced from any energy source, including th ...
... Hydrogen and electricity are often considered as complementary energy carriers for the future. Hydrogen has some unique properties, which in conjunction with electricity make it an ideal energy carrier or fuel [1,2]: • Just as electricity hydrogen can be produced from any energy source, including th ...
Silicon as an intermediary between renewable
... Furthermore, electric energy cannot be effectively stored and therefore necessitates immediate use by consumers. According to numerous experts, this future energy-carrying vehicle will be hydrogen, which produces water as the result of the highly efficient energy-delivering combustion process.2,6 In ...
... Furthermore, electric energy cannot be effectively stored and therefore necessitates immediate use by consumers. According to numerous experts, this future energy-carrying vehicle will be hydrogen, which produces water as the result of the highly efficient energy-delivering combustion process.2,6 In ...
Functional Groups
... Humic coals: derived from humic substances via a peat stage. Sapropelic coals: formed from fairly fine-grained organic muds in quiet, oxygen-deficient shallow waters. Peat: Unconsolidated, semicarbonized plant remains with high moisture content. Not true coal. ...
... Humic coals: derived from humic substances via a peat stage. Sapropelic coals: formed from fairly fine-grained organic muds in quiet, oxygen-deficient shallow waters. Peat: Unconsolidated, semicarbonized plant remains with high moisture content. Not true coal. ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.