
Stoichiometric Calculations
... or products using a balanced chemical equation. We can interpret a balanced equation in several ways. The most common way is to interpret it as indicating the number of moles of each substance. For instance, this equation, 3 H2 + N2 ® 2 NH3 can be read as: 3 moles of hydrogen gas reacts with 1 mole ...
... or products using a balanced chemical equation. We can interpret a balanced equation in several ways. The most common way is to interpret it as indicating the number of moles of each substance. For instance, this equation, 3 H2 + N2 ® 2 NH3 can be read as: 3 moles of hydrogen gas reacts with 1 mole ...
Announcements - University of Illinois Urbana
... • G(T) = R(T) intersection, equal rate of heat generation & removal, no change in T • G(T) > R(T) (G(T) line above R(T) on graph): rate of heat generation > heat removal, so reactor heats up until a steady state is reached • R(T) > G(T) (R(T) line above G(T) on graph): rate of heat generation < heat ...
... • G(T) = R(T) intersection, equal rate of heat generation & removal, no change in T • G(T) > R(T) (G(T) line above R(T) on graph): rate of heat generation > heat removal, so reactor heats up until a steady state is reached • R(T) > G(T) (R(T) line above G(T) on graph): rate of heat generation < heat ...
SCH3U0FinalExamReview - Savita Pall and Chemistry
... 13. Neutral atoms are always larger than their positive ions. As an atom becomes positive it loses electrons. It is the electrons that determine size, therefore the ...
... 13. Neutral atoms are always larger than their positive ions. As an atom becomes positive it loses electrons. It is the electrons that determine size, therefore the ...
Answers to Problem-Solving Practice Problems
... Notice that the result is expressed to three significant figures, because both the mass and the density had three significant figures. (4) Check your answer: Because the density is a little less than 1.00 g/mL, the volume in milliliters should be a little larger than the mass in grams. The calculate ...
... Notice that the result is expressed to three significant figures, because both the mass and the density had three significant figures. (4) Check your answer: Because the density is a little less than 1.00 g/mL, the volume in milliliters should be a little larger than the mass in grams. The calculate ...
Solutions - ChemConnections
... Mass balance indicates that we have the same number and type of atoms on both sides of the equation (so that mass is conserved). Similarly, net charge must also be conserved. We cannot have a buildup of charge on one side of the reaction or the other. In redox reactions, electrons are used to balanc ...
... Mass balance indicates that we have the same number and type of atoms on both sides of the equation (so that mass is conserved). Similarly, net charge must also be conserved. We cannot have a buildup of charge on one side of the reaction or the other. In redox reactions, electrons are used to balanc ...
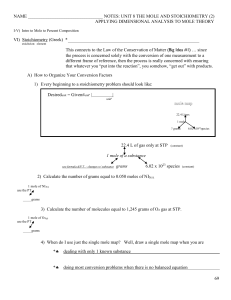
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... all you need to do is substitute into the equation the two given values and solve for the third. then, use unit cancellation to convert between grams moles AND ...
... all you need to do is substitute into the equation the two given values and solve for the third. then, use unit cancellation to convert between grams moles AND ...
Topic 1 Quantitative Chemistry Answers - slider-dpchemistry-11
... Symbol M or Mm. It is the mass per mole of a substance. The molar mass has the same numerical value as the atomic mass or molecular mass, but has the units g mol -1. For example water has a Mr of (H: 2 x 1.01) + (O: 16.00) = 18.02 g, so its molar mass, M(H2O) is 18.02 g mol -1. d) relative formula m ...
... Symbol M or Mm. It is the mass per mole of a substance. The molar mass has the same numerical value as the atomic mass or molecular mass, but has the units g mol -1. For example water has a Mr of (H: 2 x 1.01) + (O: 16.00) = 18.02 g, so its molar mass, M(H2O) is 18.02 g mol -1. d) relative formula m ...
CHAPTER 6 THERMOCHEMISTRY
... | Heat loss by hot water | = | heat gain by cooler water | The magnitudes of heat loss and heat gain are equal in calorimetry problems. The only difference is the sign (positive or negative). To avoid sign errors, keep all quantities positive and, if necessary, deduce the correct signs at the end of ...
... | Heat loss by hot water | = | heat gain by cooler water | The magnitudes of heat loss and heat gain are equal in calorimetry problems. The only difference is the sign (positive or negative). To avoid sign errors, keep all quantities positive and, if necessary, deduce the correct signs at the end of ...
CHAPTER 12 | The Chemistry of Solids
... We are to consider whether band theory can explain why hydrogen at very low temperatures and high pressures might act like a metal and conduct electricity. Analyze Band theory is a model of bonding in which orbitals on many atoms are combined, as in molecular orbital theory, to form a fully or parti ...
... We are to consider whether band theory can explain why hydrogen at very low temperatures and high pressures might act like a metal and conduct electricity. Analyze Band theory is a model of bonding in which orbitals on many atoms are combined, as in molecular orbital theory, to form a fully or parti ...
Part 3-ICHO-31-35
... PART A The standard enthalpy of formation of CO2(g) and H2O(l) at 25.00 °C are –393.51 and –285.83 kJ mol-1, respectively. The gas constant, R = 8.314 J K-1 mol-1. (Relative atomic masses : H = 1.0; C = 12.0; O = 16.0) A sample of solid Q that weighs 0.6000 g, is combusted in an excess of oxygen in ...
... PART A The standard enthalpy of formation of CO2(g) and H2O(l) at 25.00 °C are –393.51 and –285.83 kJ mol-1, respectively. The gas constant, R = 8.314 J K-1 mol-1. (Relative atomic masses : H = 1.0; C = 12.0; O = 16.0) A sample of solid Q that weighs 0.6000 g, is combusted in an excess of oxygen in ...
chapter 18 - HCC Learning Web
... Mass balance indicates that we have the same number and type of atoms on both sides of the equation (so that mass is conserved). Similarly, net charge must also be conserved. We cannot have a buildup of charge on one side of the reaction or the other. In redox equations, electrons are used to balanc ...
... Mass balance indicates that we have the same number and type of atoms on both sides of the equation (so that mass is conserved). Similarly, net charge must also be conserved. We cannot have a buildup of charge on one side of the reaction or the other. In redox equations, electrons are used to balanc ...
Chapter 1: Matter and Measurements
... (b) “A molecule is made up of nonmetal atoms” is always true, by definition. (c) “An ionic compound has at least one metal atom” is usually true. Most ionic compounds do contain a metal, but there are ionic compounds in which the cation (positive ion) is not a metal, such as in ammonium chloride, NH ...
... (b) “A molecule is made up of nonmetal atoms” is always true, by definition. (c) “An ionic compound has at least one metal atom” is usually true. Most ionic compounds do contain a metal, but there are ionic compounds in which the cation (positive ion) is not a metal, such as in ammonium chloride, NH ...
Chapter 3 Stoichiometry
... aluminum about the size of pencil eraser contains approximately 2 × 1022 aluminum atoms! Chemists use a special unit when counting the numbers of atoms or molecules in a sample that has been measured at the macroscopic level. This unit is called the mole, and it is based on quantities that we wor ...
... aluminum about the size of pencil eraser contains approximately 2 × 1022 aluminum atoms! Chemists use a special unit when counting the numbers of atoms or molecules in a sample that has been measured at the macroscopic level. This unit is called the mole, and it is based on quantities that we wor ...
FREE Sample Here
... 44) When methane, CH4, undergoes combustion with oxygen, the usual products are carbon dioxide and water. Carbon monoxide is formed when the limiting reactant is A) carbon dioxide. B) methane. C) oxygen. D) water. Answer: C Diff: 2 Topic: Section 6.5 Reactions with Limiting Amounts of Reactants 45) ...
... 44) When methane, CH4, undergoes combustion with oxygen, the usual products are carbon dioxide and water. Carbon monoxide is formed when the limiting reactant is A) carbon dioxide. B) methane. C) oxygen. D) water. Answer: C Diff: 2 Topic: Section 6.5 Reactions with Limiting Amounts of Reactants 45) ...
Section 1.3 - The Student Room
... most stable states at 1 atmosphere pressure and at a stated temperature, often 298 K). b Standard enthalpy change of formation is the enthalpy change when 1 mole of a compound is formed from its elements, with both the compound and its elements being in their standard states (ie their most stable st ...
... most stable states at 1 atmosphere pressure and at a stated temperature, often 298 K). b Standard enthalpy change of formation is the enthalpy change when 1 mole of a compound is formed from its elements, with both the compound and its elements being in their standard states (ie their most stable st ...
chapter 20 - United International College
... A galvanic cell contains an anode, which is the electrode at which oxidation occurs and a cathode at which reduction occurs. To complete the electrical circuit, the solutions must be connected by a conducting medium through which the cations and anions can move from one electrode compartment to the ...
... A galvanic cell contains an anode, which is the electrode at which oxidation occurs and a cathode at which reduction occurs. To complete the electrical circuit, the solutions must be connected by a conducting medium through which the cations and anions can move from one electrode compartment to the ...
Stoichiometry - Social Circle City Schools
... greater detail chemical formulas and chemical reactions. Specifically, in this Unit we investigate stoichiometry, the relationship between quantities of materials in chemical reactions. In Unit 4 (Chemical Reactions and Solution Stoichiometry), we will expand our study of stoichiometry to include di ...
... greater detail chemical formulas and chemical reactions. Specifically, in this Unit we investigate stoichiometry, the relationship between quantities of materials in chemical reactions. In Unit 4 (Chemical Reactions and Solution Stoichiometry), we will expand our study of stoichiometry to include di ...
Teacher Edition Calculations
... Perform a first-hand investigation to make solutions to specified volume-to-volume and massto-volume specifications and dilute them to specified concentrations (cV = constant) Calculate mass and concentration relationships in precipitation reactions as they are encountered Explain and use the equati ...
... Perform a first-hand investigation to make solutions to specified volume-to-volume and massto-volume specifications and dilute them to specified concentrations (cV = constant) Calculate mass and concentration relationships in precipitation reactions as they are encountered Explain and use the equati ...
Determination of Equilibrium Constants for Reactions between Nitric
... formation reactions took place in the bubble-column reactor mentioned above. Because of volatilization of the concentrated aqueous ammonia, a glass bottle filled with deionized water was placed to absorb NH3 to eliminate its interference before the exhaust entered the gas analyzer. The NO concentrati ...
... formation reactions took place in the bubble-column reactor mentioned above. Because of volatilization of the concentrated aqueous ammonia, a glass bottle filled with deionized water was placed to absorb NH3 to eliminate its interference before the exhaust entered the gas analyzer. The NO concentrati ...
Lab Manual (Eng. Medium)
... harmful/corrosive liquids. It is used as follows: 1. Insert the adaptor into the top end of the pipette. 2. Dip the pipette tip in the liquid. 3. Press the adaptor to force the air out and then release to suck the liquid into the pipette. 4. Fill the pipette to just above the calibration mark. 5. Ad ...
... harmful/corrosive liquids. It is used as follows: 1. Insert the adaptor into the top end of the pipette. 2. Dip the pipette tip in the liquid. 3. Press the adaptor to force the air out and then release to suck the liquid into the pipette. 4. Fill the pipette to just above the calibration mark. 5. Ad ...
2nd Semester Practice Chemistry Final 2009
... c. they can be easily compressed. d. the energy of the particles is high. 47. A substance whose water solution does NOT conduct a current is a(n) a. polar substance. c. electrolyte. b. nonelectrolyte. d. ionic substance. 48. Which of the following will dissolve most slowly? a. large salt crystals in ...
... c. they can be easily compressed. d. the energy of the particles is high. 47. A substance whose water solution does NOT conduct a current is a(n) a. polar substance. c. electrolyte. b. nonelectrolyte. d. ionic substance. 48. Which of the following will dissolve most slowly? a. large salt crystals in ...
BRIEF ANSWERS TO SELECTED PROBLEMS APPENDIX G
... His measurements showed that a gas was involved in the reaction. He called this gas oxygen (one of his key discoveries). 1.16 A well-designed experiment must have the following essential features: (1) There must be at least two variables that are expected to be related; (2) there must be a way to co ...
... His measurements showed that a gas was involved in the reaction. He called this gas oxygen (one of his key discoveries). 1.16 A well-designed experiment must have the following essential features: (1) There must be at least two variables that are expected to be related; (2) there must be a way to co ...
Equilibrium - pedagogics.ca
... The equilibrium constant provides information about how far a reaction proceeds at a particular temperature. The values of the equilibrium constants for a series of reactions at 298 K are given in Table 7.1. These equilibrium constants are all very ...
... The equilibrium constant provides information about how far a reaction proceeds at a particular temperature. The values of the equilibrium constants for a series of reactions at 298 K are given in Table 7.1. These equilibrium constants are all very ...
Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]
... 1. Combination Reactions In combination reactions two or more substances react to form one product. For example, magnesium metal burns brilliantly in air to produce magnesium oxide: 2Mg(s) + O2(g) g 2 MgO(s) This reaction is used to produce the bright flame generated by flares and some fireworks. A ...
... 1. Combination Reactions In combination reactions two or more substances react to form one product. For example, magnesium metal burns brilliantly in air to produce magnesium oxide: 2Mg(s) + O2(g) g 2 MgO(s) This reaction is used to produce the bright flame generated by flares and some fireworks. A ...
5 Steps
... Not too long ago, you enrolled in AP Chemistry. A curiosity about chemistry, encouragement from a respected teacher, or the simple fact that it was a requirement may have been your motivation. No matter what the reason, you find yourself flipping through a book, which promises to help you culminate ...
... Not too long ago, you enrolled in AP Chemistry. A curiosity about chemistry, encouragement from a respected teacher, or the simple fact that it was a requirement may have been your motivation. No matter what the reason, you find yourself flipping through a book, which promises to help you culminate ...
Thermometric titration

A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century (Bell and Cowell, 1913). In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.The applications of thermometric titrimetry discussed on this page are by no means exhaustive. The reader is referred to the bibliography for further reading on the subject.



















![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)
