History of the Atom
... • There exist empty space between atoms • Atoms are completely solid • Atoms are homogeneous with no internal structure • Atoms differ in size, shape and weight ...
... • There exist empty space between atoms • Atoms are completely solid • Atoms are homogeneous with no internal structure • Atoms differ in size, shape and weight ...
subatomic-particles
... from classical physics. But it also reflects the modern understanding that at the quantum scale matter and energy behave very differently from what much of everyday experience would lead us to expect. The idea of a particle underwent serious rethinking when experiments showed that light could behave ...
... from classical physics. But it also reflects the modern understanding that at the quantum scale matter and energy behave very differently from what much of everyday experience would lead us to expect. The idea of a particle underwent serious rethinking when experiments showed that light could behave ...
Short-Lived Resonance States
... • Other resonances have since been discovered, and although the recognition of such states is difficult their masses and spin characteristics have been measured. They all show strong nuclear decay yielding baryons (often nucleons) and mesons which are easily observed. Including these resonances the ...
... • Other resonances have since been discovered, and although the recognition of such states is difficult their masses and spin characteristics have been measured. They all show strong nuclear decay yielding baryons (often nucleons) and mesons which are easily observed. Including these resonances the ...
THE STANDARD MODEL:
... The best description of how matter and energy interact (sans gravity) is called “The Standard Model” It describes the organization of all of the particles and how they interact. The elementary particles are divided into two families called quarks and leptons. Each family consists of six particles an ...
... The best description of how matter and energy interact (sans gravity) is called “The Standard Model” It describes the organization of all of the particles and how they interact. The elementary particles are divided into two families called quarks and leptons. Each family consists of six particles an ...
The types of particle accelerator
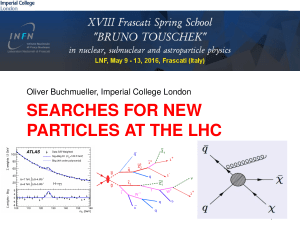
... speed of light, colliding them at high energies and recording the results on huge computers. But what is the LHC looking for? Is Science wrong? – If the LHC does not conclude any results as to the origin Extra Dimensions are there extra dimensions that we cannot see? Dark Matter – what is the nature ...
... speed of light, colliding them at high energies and recording the results on huge computers. But what is the LHC looking for? Is Science wrong? – If the LHC does not conclude any results as to the origin Extra Dimensions are there extra dimensions that we cannot see? Dark Matter – what is the nature ...
14. Elementary Particles
... much larger that the kinetic energy observed for any electron emitted from nuclei. ...
... much larger that the kinetic energy observed for any electron emitted from nuclei. ...
Elementary particles and typical scales in HEP
... For example the ration of the mass of the muon and the mass of the electron is 207, which has to be taken by hand. 2. It does not include gravity. The effects of the gravity are quite negligible in PP, however they are crucial in cosmology and in the study of the early universe. SM is a quantum theo ...
... For example the ration of the mass of the muon and the mass of the electron is 207, which has to be taken by hand. 2. It does not include gravity. The effects of the gravity are quite negligible in PP, however they are crucial in cosmology and in the study of the early universe. SM is a quantum theo ...
Chapter 11 Vocabulary 1. Atom – the smallest particle into which an
... Chapter 11 Vocabulary 1. Atom – the smallest particle into which an element can be divided and still be the same substance. 2. Electrons – the negatively charged particles found in all atoms. 3. Nucleus – the tiny, extremely dense, positively charged region in the center of the atom. 4. Electron clo ...
... Chapter 11 Vocabulary 1. Atom – the smallest particle into which an element can be divided and still be the same substance. 2. Electrons – the negatively charged particles found in all atoms. 3. Nucleus – the tiny, extremely dense, positively charged region in the center of the atom. 4. Electron clo ...
ppt - Infn
... The answer is yes and deserves a more detailed explanation. Actually, this can happen for two reasons: 1) The 0nbb decay is not caused by the exchange of the light Majorana neutrinos, but by some other mechanism. The obvious question then is how can we tell which mechanism is responsible for the 0nb ...
... The answer is yes and deserves a more detailed explanation. Actually, this can happen for two reasons: 1) The 0nbb decay is not caused by the exchange of the light Majorana neutrinos, but by some other mechanism. The obvious question then is how can we tell which mechanism is responsible for the 0nb ...
1 Elementary Particle Mass-Radius Relationships S. Reucroft* and
... an atom-like structure with two positively charged electrons in orbit around the third negatively charged electron. The centripetal force is provided by a combination of electrostatics and gravity. The proton mass is given by the effective mass of the three constituent electrons. In this paper we co ...
... an atom-like structure with two positively charged electrons in orbit around the third negatively charged electron. The centripetal force is provided by a combination of electrostatics and gravity. The proton mass is given by the effective mass of the three constituent electrons. In this paper we co ...
Atomic Structure - Sierra Vista Chemistry
... FUNDAMENTAL PROPERTIES OF MODELS A model does not equal reality. Models are oversimplifications, and are therefore often wrong. Models become more complicated as they age. ...
... FUNDAMENTAL PROPERTIES OF MODELS A model does not equal reality. Models are oversimplifications, and are therefore often wrong. Models become more complicated as they age. ...
Electric Fields
... electric force on other charged particles. Because of their force fields, charged particles can exert force on each other without actually touching. Electric fields are generally represented by arrows, as you can see in theFigure below. The arrows show the direction of electric force around a positi ...
... electric force on other charged particles. Because of their force fields, charged particles can exert force on each other without actually touching. Electric fields are generally represented by arrows, as you can see in theFigure below. The arrows show the direction of electric force around a positi ...