The Singlet-Triplet Spectroscopy of 1,3
... to the double bonds in the molecule).7 Accordingly, we have modeled the transition as a B1u type transition (Table II from Hougen6), which restricts K to 0, ±2. There will also be a contribution from a B3u type transition, but that contribution is calculated to be several orders of magnitude less i ...
... to the double bonds in the molecule).7 Accordingly, we have modeled the transition as a B1u type transition (Table II from Hougen6), which restricts K to 0, ±2. There will also be a contribution from a B3u type transition, but that contribution is calculated to be several orders of magnitude less i ...
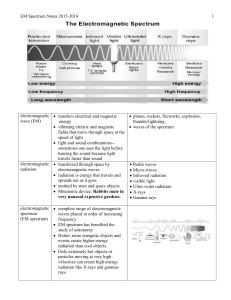
EM Spectrum Notes 2015-2016
... EM Spectrum Notes 2015-2016 blue shift objects moving towards Earth shorter wavelength ...
... EM Spectrum Notes 2015-2016 blue shift objects moving towards Earth shorter wavelength ...
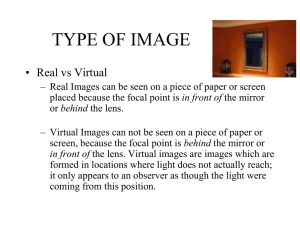
Document
... 1. Light must pass from a more optically dense to less optically dense medium. 2. There are only specific angles of incidence, called the critical angle, which is different for each medium. ...
... 1. Light must pass from a more optically dense to less optically dense medium. 2. There are only specific angles of incidence, called the critical angle, which is different for each medium. ...
04_LectureOutline
... The photoelectric effect: • When light shines on metal, electrons can be emitted • Frequency must be higher than minimum, characteristic of material • Increased frequency—more energetic electrons • Increased intensity—more electrons, same energy ...
... The photoelectric effect: • When light shines on metal, electrons can be emitted • Frequency must be higher than minimum, characteristic of material • Increased frequency—more energetic electrons • Increased intensity—more electrons, same energy ...
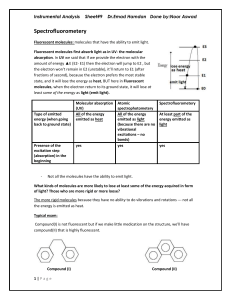
Physical chemistry exam, quiz, homework with Solution
... (A) The sulfur atom can access d-orbitals (B) Breakdown of the Pauli principle (C) Breakdown of the Born-Oppenheimer approximation (D) Excited vibrational states (E) Excited rotational states (A) 21. Which of the following is NOT a correct aspect of the Born-Oppenheimer approximation (A) The electro ...
... (A) The sulfur atom can access d-orbitals (B) Breakdown of the Pauli principle (C) Breakdown of the Born-Oppenheimer approximation (D) Excited vibrational states (E) Excited rotational states (A) 21. Which of the following is NOT a correct aspect of the Born-Oppenheimer approximation (A) The electro ...
Engineering Optics and Optical Techniques
... Ampere’s Circutal Law states that a time-varying E-field and j induces a B-field. The notion is that a time-varying E-field will be accompanied by a B-field. * o ~ 4 10 7 m kg / C 2 : magnetic permeability in vacuum…degree of measure of magnetic induction (B) for a given magnetic field stren ...
... Ampere’s Circutal Law states that a time-varying E-field and j induces a B-field. The notion is that a time-varying E-field will be accompanied by a B-field. * o ~ 4 10 7 m kg / C 2 : magnetic permeability in vacuum…degree of measure of magnetic induction (B) for a given magnetic field stren ...
BJ - Faculty Web Pages
... (d) Say you wish to study the anti-Stokes portion of the Raman spectrum of a sample, but you are having difficulty because the peaks barely rise above the baseline. What could you do to the sample to increase the intensity of the spectrum? Explain. Heat the sample. Most molecules do not change frequ ...
... (d) Say you wish to study the anti-Stokes portion of the Raman spectrum of a sample, but you are having difficulty because the peaks barely rise above the baseline. What could you do to the sample to increase the intensity of the spectrum? Explain. Heat the sample. Most molecules do not change frequ ...
The Making of Quantum Theory
... A continuous spectrum is produced when white light is passed through a prism. The result is line a rainbow, where ROYGBIV can be seen. Absorption Spectra: In order to excite an electron a certain amount of energy is required (at a particular wavelength) corresponding to the differences between the ...
... A continuous spectrum is produced when white light is passed through a prism. The result is line a rainbow, where ROYGBIV can be seen. Absorption Spectra: In order to excite an electron a certain amount of energy is required (at a particular wavelength) corresponding to the differences between the ...
sci2201-SP06-lab5-Ma..
... smallest of which is the electron. In a larger scale, the bar-magnets like the ones we are using are made up of materials that are called ferromagnetic. Iron is an example of a ferromagnetic material. The simulation titled “Magnetic Domains” illustrates this concept. It shows a piece of ferromagneti ...
... smallest of which is the electron. In a larger scale, the bar-magnets like the ones we are using are made up of materials that are called ferromagnetic. Iron is an example of a ferromagnetic material. The simulation titled “Magnetic Domains” illustrates this concept. It shows a piece of ferromagneti ...
Light and Blackbody radiation
... If it takes one hour for light to travel from Saturn to Earth, how far apart are the two planets? A. B. C. D. ...
... If it takes one hour for light to travel from Saturn to Earth, how far apart are the two planets? A. B. C. D. ...
File
... emitting light while receiving energy from another source Ex. Fluorescent light tubes Fluorescence is a combination of two different light sources, electric discharge and phosphorescence • Electricity passes along the tube and causes particles of mercury vapour to emit invisible ultraviolet (UV) ene ...
... emitting light while receiving energy from another source Ex. Fluorescent light tubes Fluorescence is a combination of two different light sources, electric discharge and phosphorescence • Electricity passes along the tube and causes particles of mercury vapour to emit invisible ultraviolet (UV) ene ...