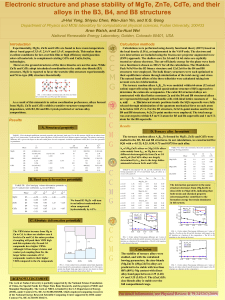
Electronic structure and phase stability of MgTe, ZnTe, CdTe, and
... wave functions is chosen as 300 eV for all the calculations. The MonkhorstPack 9x9x9 for the B3 binary structure and 12x12x6 for the B4 and B8 structures were employed. The bulk binary structures were each optimized to their equilibrium volume through minimization of the total energy and stress. The ...
... wave functions is chosen as 300 eV for all the calculations. The MonkhorstPack 9x9x9 for the B3 binary structure and 12x12x6 for the B4 and B8 structures were employed. The bulk binary structures were each optimized to their equilibrium volume through minimization of the total energy and stress. The ...
Virtual Objects on Real Oceans
... real ocean scene. A detection algorithm is applied to identify significant waves crestlines. A virtual ocean is then reconstructed using Gerstner model; its parameters are inferred and adjusted by the user to match the crestlines and to provide a smooth reconstruction between adjacent waves. An appl ...
... real ocean scene. A detection algorithm is applied to identify significant waves crestlines. A virtual ocean is then reconstructed using Gerstner model; its parameters are inferred and adjusted by the user to match the crestlines and to provide a smooth reconstruction between adjacent waves. An appl ...
Honors Biology Chapter 2 Power Point
... • What three possible atoms can make a hydrogen bond with hydrogen? • List the forces in order of strength. ...
... • What three possible atoms can make a hydrogen bond with hydrogen? • List the forces in order of strength. ...
ppt Lewis Dot Diagram Rules
... Oxygens. Step 3 Add next frequently occurring atoms around the central atom keeping symmetry in mind. •You cannot put two of one atom next to each other Ie/ OOAl must be O Al O • Step 4 Add all the electrons and then put Hydrogen on LAST, oxygen atoms on 2nd to last. ...
... Oxygens. Step 3 Add next frequently occurring atoms around the central atom keeping symmetry in mind. •You cannot put two of one atom next to each other Ie/ OOAl must be O Al O • Step 4 Add all the electrons and then put Hydrogen on LAST, oxygen atoms on 2nd to last. ...
Course Materials (These materials are for non
... repeatability - ability to reproduce a given position from the same direction (unidirectional repeatability) or from two directions (bi-directional repeatability), for multiple data points it is the distribution about the mean (standard deviation), usually the most important ...
... repeatability - ability to reproduce a given position from the same direction (unidirectional repeatability) or from two directions (bi-directional repeatability), for multiple data points it is the distribution about the mean (standard deviation), usually the most important ...
Homework 1 - Devin Gatherwright IET 307 Portfolio
... Covalent bonds are directional in nature, meaning that the bond exists between specific atoms and can only exist in the direction between one atom and the neighboring atom that takes part in the sharing of the electron. Some characteristics of covalent bonding are as follows: covalent bonding is th ...
... Covalent bonds are directional in nature, meaning that the bond exists between specific atoms and can only exist in the direction between one atom and the neighboring atom that takes part in the sharing of the electron. Some characteristics of covalent bonding are as follows: covalent bonding is th ...
File
... 1. An atom is the smallest particle of an element that retains the properties of that element. 2. The nucleus is a small, dense region located at the center of an atom. 3. The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles cal ...
... 1. An atom is the smallest particle of an element that retains the properties of that element. 2. The nucleus is a small, dense region located at the center of an atom. 3. The nucleus is made up of at least one positively charged particle called a proton and usually one or more neutral particles cal ...
Chapter 2
... Review__________– atoms share electrons ________- one atom strips electrons from other to produce ions _________ - weak, between partial opposite charges _______________weak, fleeting interactions ...
... Review__________– atoms share electrons ________- one atom strips electrons from other to produce ions _________ - weak, between partial opposite charges _______________weak, fleeting interactions ...
Atomic Structure - Hudson City School District
... What is a chemical bond? • Attraction between two or more atoms due to opposite charges • YouTube - ?Ionic and covalent bonding animation?? ...
... What is a chemical bond? • Attraction between two or more atoms due to opposite charges • YouTube - ?Ionic and covalent bonding animation?? ...
Use the following to answer questions 1-14:
... Fill in the blank with the most appropriate term from the chapter, unit, or course. To summarize what happens to substances during a chemical reaction, scientists use a chemical _______________________. Arsenic has a total of 33 electrons. It has _______________________ electron shells around its nu ...
... Fill in the blank with the most appropriate term from the chapter, unit, or course. To summarize what happens to substances during a chemical reaction, scientists use a chemical _______________________. Arsenic has a total of 33 electrons. It has _______________________ electron shells around its nu ...
p-type and n-type semiconductors
... An Atomic Description of Silicon All matter is composed of atoms. Atoms, in turn, are composed of positively charged protons, negatively charged electrons, and neutral neutrons. The protons and neutrons, which are of approximately equal size, comprise the closepacked central “nucleus” of the atom, w ...
... An Atomic Description of Silicon All matter is composed of atoms. Atoms, in turn, are composed of positively charged protons, negatively charged electrons, and neutral neutrons. The protons and neutrons, which are of approximately equal size, comprise the closepacked central “nucleus” of the atom, w ...
4.1 PPT- Atomic Theory and Bonding
... Atoms gain and lose electrons in an attempt to be STABLE. The noble gases are stable because they have FULL outer shells of electrons. They don’t need to lose or gain any e-s. Atoms in each period want to have the same number of electrons in their outer shell (VALENCE ELECTRONS) as the noble gases ...
... Atoms gain and lose electrons in an attempt to be STABLE. The noble gases are stable because they have FULL outer shells of electrons. They don’t need to lose or gain any e-s. Atoms in each period want to have the same number of electrons in their outer shell (VALENCE ELECTRONS) as the noble gases ...
lesson 5
... LESSON I How do some 5 ^ compounds form .? You have learned about electron shells. Now use this knowledge to understand how atoms link up to form compounds. Not all atoms form compounds. Only atoms that have outer shells that are not full form compounds. The elements of Group 18 have complete outer ...
... LESSON I How do some 5 ^ compounds form .? You have learned about electron shells. Now use this knowledge to understand how atoms link up to form compounds. Not all atoms form compounds. Only atoms that have outer shells that are not full form compounds. The elements of Group 18 have complete outer ...
Chapter 07 and 08 Chemical Bonding and Molecular
... • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell which is LOWER IN E ...
... • Made of 2 or more elements in a definite proportion by mass • Physically and chemically different from the elements that make up the compound • All elements (except Noble gases) react to gain a stable octet. (duet-for H through B) • Compounds form to gain a stable valence shell which is LOWER IN E ...
Practice Bypass Answers
... SC 119 PRACTICE Assessment - ANSWERS: 1. Outdoor grilling is a very popular method of cooking. Propane is the gas that is commonly used in grills. Three things are required for a gas grill to ignite: gas, oxygen from the air and a spark. When the grill is turned on, propane is delivered to the ignit ...
... SC 119 PRACTICE Assessment - ANSWERS: 1. Outdoor grilling is a very popular method of cooking. Propane is the gas that is commonly used in grills. Three things are required for a gas grill to ignite: gas, oxygen from the air and a spark. When the grill is turned on, propane is delivered to the ignit ...
September 20, 2000 - University of South Florida
... The actual atmospheric effect is much more complicated than shown above due to multiplescattering and gaseous absorption effects. The surface reflection term is also much more complicated due to solar/viewing geometry and surface roughness (Mobley, 1999). But the concept remains the same: surface-re ...
... The actual atmospheric effect is much more complicated than shown above due to multiplescattering and gaseous absorption effects. The surface reflection term is also much more complicated due to solar/viewing geometry and surface roughness (Mobley, 1999). But the concept remains the same: surface-re ...
Grain contrast_published - Chalmers University of Technology
... in the Kikuchi line patterns. Thus, the patterns do not need to be indexed; they can be constructed directly, without knowledge about the crystal structure of the material. As grain boundaries produce less band contrast they appear dark in these maps. This is illustrated in figure 2(b), which shows ...
... in the Kikuchi line patterns. Thus, the patterns do not need to be indexed; they can be constructed directly, without knowledge about the crystal structure of the material. As grain boundaries produce less band contrast they appear dark in these maps. This is illustrated in figure 2(b), which shows ...
NON-DESTRUCTIVE TESTING
... occurs at an air-metal interface, and therefore to get the ultrasonic wave into the metal a liquid (usually grease) is placed between the source and metal Pulsed beams of ultrasonic waves pass through the object ...
... occurs at an air-metal interface, and therefore to get the ultrasonic wave into the metal a liquid (usually grease) is placed between the source and metal Pulsed beams of ultrasonic waves pass through the object ...
Notes
... Indicate the oxidizing and reducing agents in each of the following reactions. Assign oxidation numbers to all atoms in the equation. For each reaction write the oxidation and reduction half reaction: ...
... Indicate the oxidizing and reducing agents in each of the following reactions. Assign oxidation numbers to all atoms in the equation. For each reaction write the oxidation and reduction half reaction: ...
The format of this test is MULTIPLE CHOICE
... 6. Which nonmetal elements can form triple bonds? Nitrogen family 7. Which nonmetal elements can only form single bond? Halogens 8. What major assumption of the VSEPR theory means that bond angles will be as large as possible and that compound will exist in 3 dimensional space? Unpaired electron clo ...
... 6. Which nonmetal elements can form triple bonds? Nitrogen family 7. Which nonmetal elements can only form single bond? Halogens 8. What major assumption of the VSEPR theory means that bond angles will be as large as possible and that compound will exist in 3 dimensional space? Unpaired electron clo ...
Ionic Bonding - petersonORHS
... gain, or share valence electrons in order to obtain a full set of eight (8) valence electrons. • Valence- refers to the outer electrons in an atom. These are the electrons on the outer shell, which is the highest energy level. ...
... gain, or share valence electrons in order to obtain a full set of eight (8) valence electrons. • Valence- refers to the outer electrons in an atom. These are the electrons on the outer shell, which is the highest energy level. ...
Low-energy electron diffraction

Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low energy electrons (20–200 eV) and observation of diffracted electrons as spots on a fluorescent screen.LEED may be used in one of two ways: Qualitatively, where the diffraction pattern is recorded and analysis of the spot positions gives information on the symmetry of the surface structure. In the presence of an adsorbate the qualitative analysis may reveal information about the size and rotational alignment of the adsorbate unit cell with respect to the substrate unit cell. Quantitatively, where the intensities of diffracted beams are recorded as a function of incident electron beam energy to generate the so-called I-V curves. By comparison with theoretical curves, these may provide accurate information on atomic positions on the surface at hand.↑























