Review topics-blog
... Chapter 8 starts with a description of ionic bond strengths as a relationship to the lattice energy of the ions. As ions are more highly charged in magnitude, the lattice energy increases hence the ionic bond strength is stronger, and then an associated trend like melting point should increase. F ...
... Chapter 8 starts with a description of ionic bond strengths as a relationship to the lattice energy of the ions. As ions are more highly charged in magnitude, the lattice energy increases hence the ionic bond strength is stronger, and then an associated trend like melting point should increase. F ...
Unit 4: Chemical Bonding Notes Chemical Bond—a mutual
... that binds the atoms together. Chemical bonds create more stable arrangements of matter. The goal of any atom is to gain, lose, or share valence electrons creating chemical bonds to provide a mor ...
... that binds the atoms together. Chemical bonds create more stable arrangements of matter. The goal of any atom is to gain, lose, or share valence electrons creating chemical bonds to provide a mor ...
Concepts for the simulation of volume and surface scattering based
... • Field tracing allows inclusion of any component with any associated modeling technique. • In order to take maximum benefit of this field tracing capability, VirtualLab™ provides a programmable component which can be used together with all other components. • Programming languages: – C# with the fu ...
... • Field tracing allows inclusion of any component with any associated modeling technique. • In order to take maximum benefit of this field tracing capability, VirtualLab™ provides a programmable component which can be used together with all other components. • Programming languages: – C# with the fu ...
Chapter 3: The Structure of Crystalline Solids
... • Common metallic crystal structures are FCC, BCC, and HCP. Coordination number and atomic packing factor are the same for both FCC and HCP crystal structures. • We can predict the density of a material, provided we know the atomic weight, atomic radius, and crystal ...
... • Common metallic crystal structures are FCC, BCC, and HCP. Coordination number and atomic packing factor are the same for both FCC and HCP crystal structures. • We can predict the density of a material, provided we know the atomic weight, atomic radius, and crystal ...
NTD_Final_Ch3-1_3-2 DOWNLOAD
... increase in the melt due to the segregation, and therefore some portion of the impurity is incorporated into the crystal. The temperature profile of the whole system will also change. All these factors influence the homogeneity and the radial and lateral doping level of the crystal. In this process, ...
... increase in the melt due to the segregation, and therefore some portion of the impurity is incorporated into the crystal. The temperature profile of the whole system will also change. All these factors influence the homogeneity and the radial and lateral doping level of the crystal. In this process, ...
Atomic Systems and Bonding
... tend to have more free electrons since these valence electrons are more loosely bound to the nucleus. In some materials like copper, the electrons are so loosely held by the atom and so close to the neighboring atoms that it is difficult to determine which electron belongs to which atom! Under norma ...
... tend to have more free electrons since these valence electrons are more loosely bound to the nucleus. In some materials like copper, the electrons are so loosely held by the atom and so close to the neighboring atoms that it is difficult to determine which electron belongs to which atom! Under norma ...
Contribution of Structure and Morphology of Design Constituents to Performance Improvement of Multilayer Polaritonic Photodetector
... island film (∼100 nm) as an overlayer. Deposition of ultrathin metal layer of Cr (∼3 nm) was used for better adhesion for Au. For electronic sensitization of the SBH interface, it was subjected to passivation through the hydrothermal oxidation or/and the sulfur treatment. Basic (routine) detection t ...
... island film (∼100 nm) as an overlayer. Deposition of ultrathin metal layer of Cr (∼3 nm) was used for better adhesion for Au. For electronic sensitization of the SBH interface, it was subjected to passivation through the hydrothermal oxidation or/and the sulfur treatment. Basic (routine) detection t ...
An element`s properties depend on the structure of its atoms
... • A ball bouncing down a flight of stairs provides an analogy for energy levels of electrons, because the ball can come to rest only on each step, not between steps. • It’s a quantized event! ...
... • A ball bouncing down a flight of stairs provides an analogy for energy levels of electrons, because the ball can come to rest only on each step, not between steps. • It’s a quantized event! ...
Atomic Systems and Bonding
... tend to have more free electrons since these valence electrons are more loosely bound to the nucleus. In some materials like copper, the electrons are so loosely held by the atom and so close to the neighboring atoms that it is difficult to determine which electron belongs to which atom! Under norma ...
... tend to have more free electrons since these valence electrons are more loosely bound to the nucleus. In some materials like copper, the electrons are so loosely held by the atom and so close to the neighboring atoms that it is difficult to determine which electron belongs to which atom! Under norma ...
Reflection
... About 4% of the light energy is reflected at an air-glass interface. • Significant if one side is darker ...
... About 4% of the light energy is reflected at an air-glass interface. • Significant if one side is darker ...
Experimental Signatures of Topological Insulators
... topological surface states can still develop in between, but the coexistence of bulk and surface state is an issue at least in transport experiments. For this reason, it was suggested early on [6] that a strain-gap between the two Γ8 bands could be open when HgTe is grown on a CdTe substrate which l ...
... topological surface states can still develop in between, but the coexistence of bulk and surface state is an issue at least in transport experiments. For this reason, it was suggested early on [6] that a strain-gap between the two Γ8 bands could be open when HgTe is grown on a CdTe substrate which l ...
Computer simulation by quantum mechanical time dependent wave
... Budapest University of Technology and Economics, Department of Physics, Budafoki út. 8, Budapest, Hungary, H1111 ...
... Budapest University of Technology and Economics, Department of Physics, Budafoki út. 8, Budapest, Hungary, H1111 ...
chapter 2 - Scranton Prep Biology
... 6. Electron configuration and chemical properties An atom's electronconfigurationdeterminesits chemicalbehavior. ' Electron conJiguration= Distributionof electronsin an atom's electron shells The first l8 elementsof a periodicchartare arrangedsequentially by atomic numberinto threerows (periods).In ...
... 6. Electron configuration and chemical properties An atom's electronconfigurationdeterminesits chemicalbehavior. ' Electron conJiguration= Distributionof electronsin an atom's electron shells The first l8 elementsof a periodicchartare arrangedsequentially by atomic numberinto threerows (periods).In ...
on Plasma-Wall Interactions
... - The sheath potential drop, Vsh exceeds the breakdown/arc voltage. - Plasma can support sufficiently large arc current to form the spot, for example, by evaporating of wall materials or producing thermionic or field emission. • Unipolar arcs also occur when walls are made from micro-engineered mate ...
... - The sheath potential drop, Vsh exceeds the breakdown/arc voltage. - Plasma can support sufficiently large arc current to form the spot, for example, by evaporating of wall materials or producing thermionic or field emission. • Unipolar arcs also occur when walls are made from micro-engineered mate ...
Review - Final Exam
... 11. Calculate the Rf values for the chromatograph below. Show your work. Solvent line ...
... 11. Calculate the Rf values for the chromatograph below. Show your work. Solvent line ...
File
... • An atom is the smallest particle of an element that still has the properties of that element 50 million atoms, lined up end to end = 1 cm An atom = proton(s) + neutron(s) + electron(s) ...
... • An atom is the smallest particle of an element that still has the properties of that element 50 million atoms, lined up end to end = 1 cm An atom = proton(s) + neutron(s) + electron(s) ...
1. Select the correct statement about subatomic particles. a
... e. It represents a molecule made of 1 carbon atom, 2 hydrogen atoms, and 6 oxygen atoms. 24. Select the correct statement about the formula K2O. a. It represents a molecule of potassium oxide. b. It represents a substance composed of potassium atoms and oxygen atoms. c. It represents a substance con ...
... e. It represents a molecule made of 1 carbon atom, 2 hydrogen atoms, and 6 oxygen atoms. 24. Select the correct statement about the formula K2O. a. It represents a molecule of potassium oxide. b. It represents a substance composed of potassium atoms and oxygen atoms. c. It represents a substance con ...
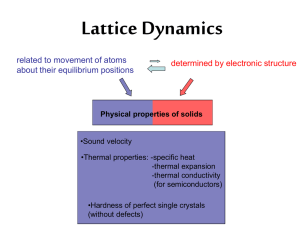
Introduction to Lattice Dynamics
... Uniform Solid Material There is energy associated with the vibrations of atoms. ...
... Uniform Solid Material There is energy associated with the vibrations of atoms. ...
PROJECT CLIL
... Free electron are negative electric charge while hole are positive electric charge ...
... Free electron are negative electric charge while hole are positive electric charge ...
Prominent 5d-orbital contribution to the conduction electrons in gold
... topology, is thought to be almost equivalent. For instance, it is well known that solid gold is very stable under many circumstances, whereas solid silver is gradually oxidized in air. The valence-band electronic configurations of these solids per atom have so far been recognized to be composed of f ...
... topology, is thought to be almost equivalent. For instance, it is well known that solid gold is very stable under many circumstances, whereas solid silver is gradually oxidized in air. The valence-band electronic configurations of these solids per atom have so far been recognized to be composed of f ...
Manufacturing Processes - Philadelphia University Jordan
... • For FCC, the coordination number is 12; the front face atom has four corner nearest-neighbor atoms surrounding it, four face atoms that are in contact from behind, and four other equivalent face atoms residing in the next unit cell to the front. • For the BCC, the coordination number is 8; each ce ...
... • For FCC, the coordination number is 12; the front face atom has four corner nearest-neighbor atoms surrounding it, four face atoms that are in contact from behind, and four other equivalent face atoms residing in the next unit cell to the front. • For the BCC, the coordination number is 8; each ce ...
Optical Analogues for Massless Dirac Particles and Conical
... Gaussian beam with a wavelength of 633 nm, covering about 5 waveguides, straight into the array. The light used for the excitation was linearly polarized in a direction perpendicular to the lattice plane. A simulation and the associated experimentally obtained fluorescence image are shown in Figs. 3 ...
... Gaussian beam with a wavelength of 633 nm, covering about 5 waveguides, straight into the array. The light used for the excitation was linearly polarized in a direction perpendicular to the lattice plane. A simulation and the associated experimentally obtained fluorescence image are shown in Figs. 3 ...
Low-energy electron diffraction

Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low energy electrons (20–200 eV) and observation of diffracted electrons as spots on a fluorescent screen.LEED may be used in one of two ways: Qualitatively, where the diffraction pattern is recorded and analysis of the spot positions gives information on the symmetry of the surface structure. In the presence of an adsorbate the qualitative analysis may reveal information about the size and rotational alignment of the adsorbate unit cell with respect to the substrate unit cell. Quantitatively, where the intensities of diffracted beams are recorded as a function of incident electron beam energy to generate the so-called I-V curves. By comparison with theoretical curves, these may provide accurate information on atomic positions on the surface at hand.↑