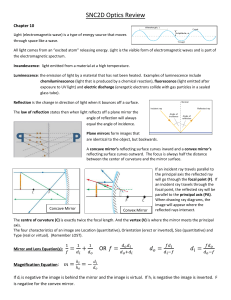
SNC2D Optics Review
... Light (electromagnetic wave) is a type of energy source that moves through space like a wave. All light comes from an “excited atom” releasing energy. Light is the visible form of electromagnetic waves and is part of the electromagnetic spectrum. Incandescence: light emitted from a material at a hig ...
... Light (electromagnetic wave) is a type of energy source that moves through space like a wave. All light comes from an “excited atom” releasing energy. Light is the visible form of electromagnetic waves and is part of the electromagnetic spectrum. Incandescence: light emitted from a material at a hig ...
Chapter 35
... in the same spatial location at the same time, and therefore overlap each other. Then one should add up their amplitudes at each spatial location and time moment. For water waves, sound waves, or waves on a rope or membrane, amplitudes are just displacements. Light waves are electromagnetic waves, s ...
... in the same spatial location at the same time, and therefore overlap each other. Then one should add up their amplitudes at each spatial location and time moment. For water waves, sound waves, or waves on a rope or membrane, amplitudes are just displacements. Light waves are electromagnetic waves, s ...
Name:
... A “bit” of extra information to “byte” off about binary numbers: In any form of communication in binary signals (like a DVD, a CD, or an optical signal through an optical fiber), the smallest unit of information is a “bit” -- which would be a “one” (a light pulse) or a “zero” (no light pulse) in a g ...
... A “bit” of extra information to “byte” off about binary numbers: In any form of communication in binary signals (like a DVD, a CD, or an optical signal through an optical fiber), the smallest unit of information is a “bit” -- which would be a “one” (a light pulse) or a “zero” (no light pulse) in a g ...
Study guide for Tools of Astronomy
... resulting in a much smaller telescope that produces much clearer images. The reflecting telescope was invented by Isaac Newton in 1669. Non-optical telescopes view all portions of the EM spectrum except for visible light portion (radio, microwave, infrared, ultraviolet, x-ray, and gamma ray) Know th ...
... resulting in a much smaller telescope that produces much clearer images. The reflecting telescope was invented by Isaac Newton in 1669. Non-optical telescopes view all portions of the EM spectrum except for visible light portion (radio, microwave, infrared, ultraviolet, x-ray, and gamma ray) Know th ...
Electronic Structure - Chemistry Teaching Resources
... or absorption of one mole of photons as follows: ...
... or absorption of one mole of photons as follows: ...
Two-Photon Excited Fluorescence Microscopy - Spectra
... objective, a simple and compact, linear prism compressor is enough. In commercial systems where highly dispersive elements such as electro-optic or acousto-optic modulators are used, a compressor with much higher dispersion compensation range is required.15, 16 By using equations (1) and (2), the di ...
... objective, a simple and compact, linear prism compressor is enough. In commercial systems where highly dispersive elements such as electro-optic or acousto-optic modulators are used, a compressor with much higher dispersion compensation range is required.15, 16 By using equations (1) and (2), the di ...
Photochemical reactions and on
... of silicon using KOH is anisotropic, and would have produced undesirable shapes (e.g. sharp corners) at the turns of the reaction channel that could induce crystallization. This prototype has a single inlet and a single outlet. Nonetheless, multiple inlets and outlets would not be difficult to produ ...
... of silicon using KOH is anisotropic, and would have produced undesirable shapes (e.g. sharp corners) at the turns of the reaction channel that could induce crystallization. This prototype has a single inlet and a single outlet. Nonetheless, multiple inlets and outlets would not be difficult to produ ...
Evidence from the Absorption and Emission Spectra
... strength f = 0.26 has been determined. The progression of the vibrational fine structure falls in the range 1050-1500 cm-l but is random. Apparently either there is more than one vibrational mode involved or the band is composed of two electronic transitions. The weak absorptions in the visible are ...
... strength f = 0.26 has been determined. The progression of the vibrational fine structure falls in the range 1050-1500 cm-l but is random. Apparently either there is more than one vibrational mode involved or the band is composed of two electronic transitions. The weak absorptions in the visible are ...
Playing with Light
... during week 9 (your TA will set the exact due date). Introduction: Light, electromagnetic radiation, plays an important role in many chemistry experiments. In spectroscopy, light is shined on or through a chemical sample and then detectors measure how the light is changed. Spectroscopy is one of the ...
... during week 9 (your TA will set the exact due date). Introduction: Light, electromagnetic radiation, plays an important role in many chemistry experiments. In spectroscopy, light is shined on or through a chemical sample and then detectors measure how the light is changed. Spectroscopy is one of the ...
Quantum Chemistry and Spectroscopy
... are on the UV range. Well we know that, most of organic compounds are transparent. And UV radiation, especially strong UV, will broke most molecules. (Visible light 400 -700 nm.) The table contain the lowest excitation and there are other transitions higher in energy. The chromophores below are simp ...
... are on the UV range. Well we know that, most of organic compounds are transparent. And UV radiation, especially strong UV, will broke most molecules. (Visible light 400 -700 nm.) The table contain the lowest excitation and there are other transitions higher in energy. The chromophores below are simp ...
Physical Chemistry - School of Chemistry, University of Leeds
... Physical chemistry is a quantitative science. Skill in practical work is as important as a knowledge of theory. Many important discoveries have resulted from observations of small inconsistencies in repeated measurements or minor discrepancies between theory and experiment. Well known examples are t ...
... Physical chemistry is a quantitative science. Skill in practical work is as important as a knowledge of theory. Many important discoveries have resulted from observations of small inconsistencies in repeated measurements or minor discrepancies between theory and experiment. Well known examples are t ...
Answers
... C) speed has no effect on the pattern It is easier to see interference if the wavelength is large. Therefore the electrons must be moving slowly. 9) The photo below shows the interference pattern produced by an electron double-slit experiment. The electrons were sent through a double-slit apparatus ...
... C) speed has no effect on the pattern It is easier to see interference if the wavelength is large. Therefore the electrons must be moving slowly. 9) The photo below shows the interference pattern produced by an electron double-slit experiment. The electrons were sent through a double-slit apparatus ...
Problems
... follow the Fermi-Dirac distribution, and they have half-integer spins. Now imagine we have a metal with n = 1023 /cm3 electrons in a cubic box of side L, and we know that electrons are Fermions. Assume the electrons are completely free to move around in the box, meaning there are no atoms in their w ...
... follow the Fermi-Dirac distribution, and they have half-integer spins. Now imagine we have a metal with n = 1023 /cm3 electrons in a cubic box of side L, and we know that electrons are Fermions. Assume the electrons are completely free to move around in the box, meaning there are no atoms in their w ...
VALIDITY OF HENRY`S LAW IN DILUTE SOLUTIONS (l)
... as for Xl = 0 the value of }' is always greater than unity and decreases with increasing Xl. This situation can only be achieved if both B' and B" are negative quantities from which it follows that in case of positive deviation for both solvents B' x' and B"x" have indeed to be subtracted from each ...
... as for Xl = 0 the value of }' is always greater than unity and decreases with increasing Xl. This situation can only be achieved if both B' and B" are negative quantities from which it follows that in case of positive deviation for both solvents B' x' and B"x" have indeed to be subtracted from each ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.