... wavelength of 632.8 nm. Never look directly at a laser beam nor permit anyone else to do so! Exposure to the direct or reflected beam for more than a few seconds will cause serious eye damage. Do not pick up the lasers and shine them around the room. If these simple precautions are taken then there ...
Introduction to Fluorescence Spectroscopies I. Theory
... suggested by A. Jablonski (1935) (see Fig. 1). The ground, first, and second electronic states are depicted by S0, S1, S2, …, respectively. The letter S (in this context) indicates a singlet excited state, in which the electrons remain paired (total spin = 0) during the excitation. In a triplet sta ...
... suggested by A. Jablonski (1935) (see Fig. 1). The ground, first, and second electronic states are depicted by S0, S1, S2, …, respectively. The letter S (in this context) indicates a singlet excited state, in which the electrons remain paired (total spin = 0) during the excitation. In a triplet sta ...
Reduction of ocular chromatic aberration by a blue light filtering
... 3. Manzanera S, Piers P, Weeber H, Artal P. Visual benefit of the combined correction of spherical and chromatic aberrations. Invest Ophthalmol Vis Sci. 2007;48:1513. ...
... 3. Manzanera S, Piers P, Weeber H, Artal P. Visual benefit of the combined correction of spherical and chromatic aberrations. Invest Ophthalmol Vis Sci. 2007;48:1513. ...
Problems - Department of Chemistry HKU
... What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study (M.J. Haugh and D.R. Dalton, J. Amer. Chem. Soc. 97, 5674 (1975)), high press ...
... What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study (M.J. Haugh and D.R. Dalton, J. Amer. Chem. Soc. 97, 5674 (1975)), high press ...
Einstein`s Postulate of Relaavity
... up (as seen by the train conductor) with a velocity of 0.4c. What is the velocity of the ball right ater it was launched as seen by an observer standing on the bridge? A]ach reference frame ...
... up (as seen by the train conductor) with a velocity of 0.4c. What is the velocity of the ball right ater it was launched as seen by an observer standing on the bridge? A]ach reference frame ...
X-ray photoelectron spectroscopy - An introduction
... A surface is really the interface between different phases (solid, liquid or gas). We can think of the surface as the top layer of atoms but in reality the state of this layer is very much influenced by the 2 – 10 atomic layers below it (~0.5 – 3 nm). Surface modification treatments are often in the ...
... A surface is really the interface between different phases (solid, liquid or gas). We can think of the surface as the top layer of atoms but in reality the state of this layer is very much influenced by the 2 – 10 atomic layers below it (~0.5 – 3 nm). Surface modification treatments are often in the ...
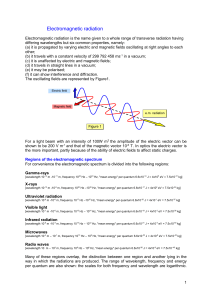
Electromagnetic radiation
... [wavelength 10-14 m -10-11 m, frequency 1022 Hz – 1019 Hz, "mean energy" per quantum 6.6x10-14 J = 4x105 eV = 7.5x10-31 kg] ...
... [wavelength 10-14 m -10-11 m, frequency 1022 Hz – 1019 Hz, "mean energy" per quantum 6.6x10-14 J = 4x105 eV = 7.5x10-31 kg] ...
Lecture 3. Fluorescence microscopy I
... Oscillator strength is integral of the absorption band Sum rule: oscillator strength, f, for one electron over all transitions is: 1x 10-16 cm2 eV ...
... Oscillator strength is integral of the absorption band Sum rule: oscillator strength, f, for one electron over all transitions is: 1x 10-16 cm2 eV ...
Optics-Diffraction - The Wave Nature of Light
... When two waves arrive at a single point in space the rule for combining the effects of the two waves together is remarkably simple and is called the Principle of Liner Superposition, which states that the amplitude of the resulting wave is the sum of the amplitudes of the two arriving waves at every ...
... When two waves arrive at a single point in space the rule for combining the effects of the two waves together is remarkably simple and is called the Principle of Liner Superposition, which states that the amplitude of the resulting wave is the sum of the amplitudes of the two arriving waves at every ...
GC-Final-Review-2014
... c. overlapping of p orbitals d. more than 1 way of drawing Lewis Structure e. metal transfers electron(s) to nonmetal f. sharing 4 or 6 electrons to complete octets g. atoms are surrounded by 8 valence electrons h. nonmetal shares electrons with another nonmetal i. unequal sharing of electrons ...
... c. overlapping of p orbitals d. more than 1 way of drawing Lewis Structure e. metal transfers electron(s) to nonmetal f. sharing 4 or 6 electrons to complete octets g. atoms are surrounded by 8 valence electrons h. nonmetal shares electrons with another nonmetal i. unequal sharing of electrons ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.