THErmAl mAss AND INsulATIoN for TEmPErATE ClImATEs
... For walls, sandwich panels of precast concrete and foam insulation provide an excellent design solution. With the insulation built in, these panels contain enough thermal mass to maintain internal building temperatures at the average summer and winter condition. This is ideal for applications such a ...
... For walls, sandwich panels of precast concrete and foam insulation provide an excellent design solution. With the insulation built in, these panels contain enough thermal mass to maintain internal building temperatures at the average summer and winter condition. This is ideal for applications such a ...
Worksheet – Measuring Heat
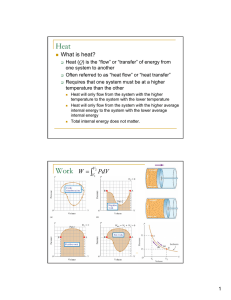
... 4. If the temperature of 34.4 g of ethanol increases from 25.0oC to 78.8oC, how much heat has been absorbed by the ethanol? (4.52 x 103 J) 5. A 4.50g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25.0oC? The specific heat of gol ...
... 4. If the temperature of 34.4 g of ethanol increases from 25.0oC to 78.8oC, how much heat has been absorbed by the ethanol? (4.52 x 103 J) 5. A 4.50g nugget of pure gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25.0oC? The specific heat of gol ...
ExamView - sample-Questions-ch10-11-12
... Identify the choice that best completes the statement or answers the question. 8. When a wool blanket is used to keep warm, what is the primary insulating material? a. wool b. air c. the trim around the edge of the blanket d. a thin layer of aluminum foil (usually not apparent) inside the blanket ...
... Identify the choice that best completes the statement or answers the question. 8. When a wool blanket is used to keep warm, what is the primary insulating material? a. wool b. air c. the trim around the edge of the blanket d. a thin layer of aluminum foil (usually not apparent) inside the blanket ...
Homework #1: Energy Unit Conversions
... Solve the following problems using the conversions listed below. Report your answer with the correct units. Conversions: 1 calorie = 4.18 joules 1 Calorie = 1000 calories 1kilocalorie= 1 Calorie (food) 1. How many calories are in 16.00 joules? ...
... Solve the following problems using the conversions listed below. Report your answer with the correct units. Conversions: 1 calorie = 4.18 joules 1 Calorie = 1000 calories 1kilocalorie= 1 Calorie (food) 1. How many calories are in 16.00 joules? ...
Molar Heat of VaporizationREV
... solidifies (or freezes) to a solid q = mol x Hsolid. (no temperature change) ...
... solidifies (or freezes) to a solid q = mol x Hsolid. (no temperature change) ...
Dynamic insulation

Dynamic insulation is a form of insulation where cool outside air flowing through the thermal insulation in the envelope of a building will pick up heat from the insulation fibres. Buildings can be designed to exploit this to reduce the transmission heat loss (U-value) and to provide pre-warmed, draft free air to interior spaces. This is known as dynamic insulation since the U-value is no longer constant for a given wall or roof construction but varies with the speed of the air flowing through the insulation (climate adaptive building shell). Dynamic insulation is different from breathing walls. The positive aspects of dynamic insulation need to be weighed against the more conventional approach to building design which is to create an airtight envelope and provide appropriate ventilation using either natural ventilation or mechanical ventilation with heat recovery. The air-tight approach to building envelope design, unlike dynamic insulation, results in a building envelope that provides a consistent performance in terms of heat loss and risk of interstitial condensation that is independent of wind speed and direction. Under certain wind conditions a dynamically insulated building can have a higher heat transmission loss than an air-tight building with the same thickness of insulation.