AtomTest
... • The atomic number is located above the symbol in any square in the periodic table of elements. • Good luck! ...
... • The atomic number is located above the symbol in any square in the periodic table of elements. • Good luck! ...
PreAP Chemistry
... _______________ are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. _______________ _______________ are all the elements in group 1 except hydrogen, and are very reactive. _______________ _______________ metals are in g ...
... _______________ are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. _______________ _______________ are all the elements in group 1 except hydrogen, and are very reactive. _______________ _______________ metals are in g ...
Periodic Trends Worksheet
... 28. Based on the concept of periodic trends, answer the following questions for these atoms: Li, Be, Mg, Na. Be able to defend your answers. a. Which element has the highest first ionization energy? ____________________________ b. Which element has the lowest electronegativity? ____________________ ...
... 28. Based on the concept of periodic trends, answer the following questions for these atoms: Li, Be, Mg, Na. Be able to defend your answers. a. Which element has the highest first ionization energy? ____________________________ b. Which element has the lowest electronegativity? ____________________ ...
Periodic Trends Review Sheet
... 8. Define ionization energy. 9. Is it easier to form a positive ion with an element that has a high ionization energy or an element that has a low ionization energy? Why? 10. Na+ and Mg2+ ions each have ten electrons surrounding their nuclei. Which ion would you expect to have the larger radius? Exp ...
... 8. Define ionization energy. 9. Is it easier to form a positive ion with an element that has a high ionization energy or an element that has a low ionization energy? Why? 10. Na+ and Mg2+ ions each have ten electrons surrounding their nuclei. Which ion would you expect to have the larger radius? Exp ...
Unit 3 - The Periodic Table
... Found in the ___________ of the periodic table (the D block) Form ___________________ in solution (ex: Cu is bright blue when dissolved in water) This concept is ALWAYS on the REGENTS EXAM!!! Tend to be _____________________ will lose electrons or gain them depending on what other ________ ...
... Found in the ___________ of the periodic table (the D block) Form ___________________ in solution (ex: Cu is bright blue when dissolved in water) This concept is ALWAYS on the REGENTS EXAM!!! Tend to be _____________________ will lose electrons or gain them depending on what other ________ ...
The Periodic Table
... shell for its electrons. All of the elements in the second row (the second period) have two orbitals (shells) for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals (shells) for any element is seven. ...
... shell for its electrons. All of the elements in the second row (the second period) have two orbitals (shells) for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals (shells) for any element is seven. ...
REVIEW Through Course Task
... you move to the right. Therefore the MOST METALLIC ELEMENTS would be found on the ________ side of the periodic table while the LEAST METALLIC ELEMENTS would be found on the LEFT ____________ side of the periodic table. RIGHT 10. While metals come in many colors, most at the far left side of the per ...
... you move to the right. Therefore the MOST METALLIC ELEMENTS would be found on the ________ side of the periodic table while the LEAST METALLIC ELEMENTS would be found on the LEFT ____________ side of the periodic table. RIGHT 10. While metals come in many colors, most at the far left side of the per ...
Periodic Table
... While it was the first periodic table, Mendeleev had very different elements, such as the very reactive potassium and the very stable copper, in the same family. Forty years later Moseley rearranged the elements by their atomic number which gave the table better periodicity. Mendeleev ...
... While it was the first periodic table, Mendeleev had very different elements, such as the very reactive potassium and the very stable copper, in the same family. Forty years later Moseley rearranged the elements by their atomic number which gave the table better periodicity. Mendeleev ...
Lesson 1 - Scientist in Residence
... This lab introduces the periodic table, the structure of the atom, and how the positions of the elements in the periodic table relate to conduction and insulation. An element is a piece of matter in its simplest form. All matter (solid, liquid, gas) is made of atoms. Atoms join together to make mole ...
... This lab introduces the periodic table, the structure of the atom, and how the positions of the elements in the periodic table relate to conduction and insulation. An element is a piece of matter in its simplest form. All matter (solid, liquid, gas) is made of atoms. Atoms join together to make mole ...
Ch. 6 - The Periodic Table
... Most are ductile, malleable and good conductors of heat and electricity. Compounds of transition metals tend to have color. One or two valence electrons. Obtained from mineral deposits (ores) in the earth’s crust (smelting). Precious metals are used for currency among other things. Darmstadtium Vide ...
... Most are ductile, malleable and good conductors of heat and electricity. Compounds of transition metals tend to have color. One or two valence electrons. Obtained from mineral deposits (ores) in the earth’s crust (smelting). Precious metals are used for currency among other things. Darmstadtium Vide ...
Unit One Periodicity of Elements and their Properties
... 2-The atomic number of each element in Moseley‟s periodic table increases by .................. ….from the preceding element in the same ………………………… 3- ………………….., and …………………. scientists modified the Mendeleev‟s table. 4-In Mendeleev‟s table arranged the elements are arranged in …………………according to t ...
... 2-The atomic number of each element in Moseley‟s periodic table increases by .................. ….from the preceding element in the same ………………………… 3- ………………….., and …………………. scientists modified the Mendeleev‟s table. 4-In Mendeleev‟s table arranged the elements are arranged in …………………according to t ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... same time as Lothar Meyer (German chemist); these periodic tables were identical, but because Mendeleev published his table first and was able to explain its periodic trends better his was given the credit. ...
... same time as Lothar Meyer (German chemist); these periodic tables were identical, but because Mendeleev published his table first and was able to explain its periodic trends better his was given the credit. ...
Atomic Structure - RHS Encore Academy
... 16. What are metalloids? (page 136) 17. What major change occurs as you move from left to right across the periodic table? (page 138) 18. What is a valence electron? Draw a SQUARE around each valence electron in the picture! (page 139) ...
... 16. What are metalloids? (page 136) 17. What major change occurs as you move from left to right across the periodic table? (page 138) 18. What is a valence electron? Draw a SQUARE around each valence electron in the picture! (page 139) ...
The Periodic Table
... are non-metals, while the other elements are metals. • Moving from left to right across a period, elements become less metallic and exhibit more of the non-metal properties. • There is also a variation in metallic character within groups. Looking at group 14, Carbon is a non-metal, where tin and lea ...
... are non-metals, while the other elements are metals. • Moving from left to right across a period, elements become less metallic and exhibit more of the non-metal properties. • There is also a variation in metallic character within groups. Looking at group 14, Carbon is a non-metal, where tin and lea ...
Periodic Table
... When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Elements with similar chemical and physical properties are in the same column or group in the PT. ...
... When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Elements with similar chemical and physical properties are in the same column or group in the PT. ...
Unit 3 Periodic Table Vocabulary
... Alkali Metals - Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium. Sentence: You can find alkali metals in the periodic table. ...
... Alkali Metals - Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium. Sentence: You can find alkali metals in the periodic table. ...
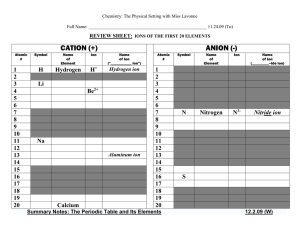
IONS OF THE FIRST 20 ELEMENTS
... The alkali metals, found in group 1 of the periodic table (formerly known as group IA), are very reactive metals that do not occur freely in nature. These metals have only one electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements. As ...
... The alkali metals, found in group 1 of the periodic table (formerly known as group IA), are very reactive metals that do not occur freely in nature. These metals have only one electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements. As ...
The representative Elements: Groups 1A – 4A
... melting point in the group, and the least reactive • All boron compounds are covalent molecules; • Boron molecules, such as BF3, have incomplete octet and acts as Lewis acid, example: BF3 + :NH3 F3B:NH3 • Boron hydrides acquire octet by forming H-bridges; • Boron oxide, B2O3, forms weak boric acid ...
... melting point in the group, and the least reactive • All boron compounds are covalent molecules; • Boron molecules, such as BF3, have incomplete octet and acts as Lewis acid, example: BF3 + :NH3 F3B:NH3 • Boron hydrides acquire octet by forming H-bridges; • Boron oxide, B2O3, forms weak boric acid ...
Study Guide for Electrons Mini-Test - seys
... - placed in tungsten filament light bulbs because it will not react with the hot filament ...
... - placed in tungsten filament light bulbs because it will not react with the hot filament ...
1 CHAPTER 5 – THE PERIODIC LAW What types of useful
... A. Before the Periodic Table was invented, about 63 elements were known. However, they were not organized and only random properties were known about each of the elements. Scientist (who are always looking for patterns) wanted to organize these. B. Dmitri Mendeleev – he made cards for all 63 known e ...
... A. Before the Periodic Table was invented, about 63 elements were known. However, they were not organized and only random properties were known about each of the elements. Scientist (who are always looking for patterns) wanted to organize these. B. Dmitri Mendeleev – he made cards for all 63 known e ...
1 CHAPTER 5 – THE PERIODIC LAW What types of useful
... A. Before the Periodic Table was invented, about 63 elements were known. However, they were not organized and only random properties were known about each of the elements. Scientist (who are always looking for patterns) wanted to organize these. B. Dmitri Mendeleev – he made cards for all 63 known e ...
... A. Before the Periodic Table was invented, about 63 elements were known. However, they were not organized and only random properties were known about each of the elements. Scientist (who are always looking for patterns) wanted to organize these. B. Dmitri Mendeleev – he made cards for all 63 known e ...
Periodic Table Assessment Quiz 2016
... element, but they do share characteristics. They both like to make four bonds and they are both located in the same column of the periodic table. Other elements in this column are germanium (Ge), tin (Sn), and lead (Pb). These other three elements are not as similar as carbon and silicon. ...
... element, but they do share characteristics. They both like to make four bonds and they are both located in the same column of the periodic table. Other elements in this column are germanium (Ge), tin (Sn), and lead (Pb). These other three elements are not as similar as carbon and silicon. ...
Unit 3 `Atoms and the Periodic Table` Study Guide
... known elements. Products – the results of a chemical reaction Proton – a subatomic particle identical with the nucleus of the hydrogen atom; found with neutrons in all atomic nuclei; carries a positive charge. Reactants – the starting materials for a reaction Replacement Reaction – chemical reaction ...
... known elements. Products – the results of a chemical reaction Proton – a subatomic particle identical with the nucleus of the hydrogen atom; found with neutrons in all atomic nuclei; carries a positive charge. Reactants – the starting materials for a reaction Replacement Reaction – chemical reaction ...